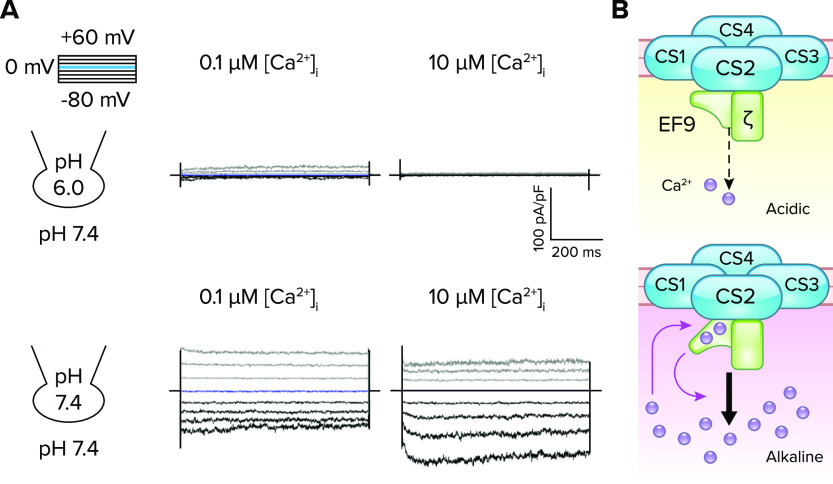
FIGURE 4.
pH-dependent Ca2+ sensing by EFCAB9 to modulate CatSper channel activity A: Ca2+-dependent potentiation of the CatSper current (ICatSper) in wild-type sperm under an intracellular alkaline condition. The traces are reproduced from Ref. 8, with permission from Cell. Inward ICatSper is minimally affected by increasing Ca2+ concentration ([Ca2+]i) under an acidic condition (pHi 6.0; top), but greatly potentiated in a [Ca2+]i-dependent manner under an alkaline condition (pHi 7.4; bottom). B: a schematic cartoon of pH-dependent Ca2+-sensing of EFCAB9 in regulating CatSper channel activity. In acidic intracellular pH, EFCAB9 (EF9) and its binding partner, CATSPERζ (ζ), form a tight binary complex and respond marginally to increasing Ca2+ (top). After intracellular alkalinization, EFCAB9-CATSPERζ interactions weaken, which enhances Ca2+ sensitivity of EFCAB9 (bottom). Ca2+ binding to EF-hand domains changes EFCAB9 conformation to fully activate CatSper.