Figure 6.
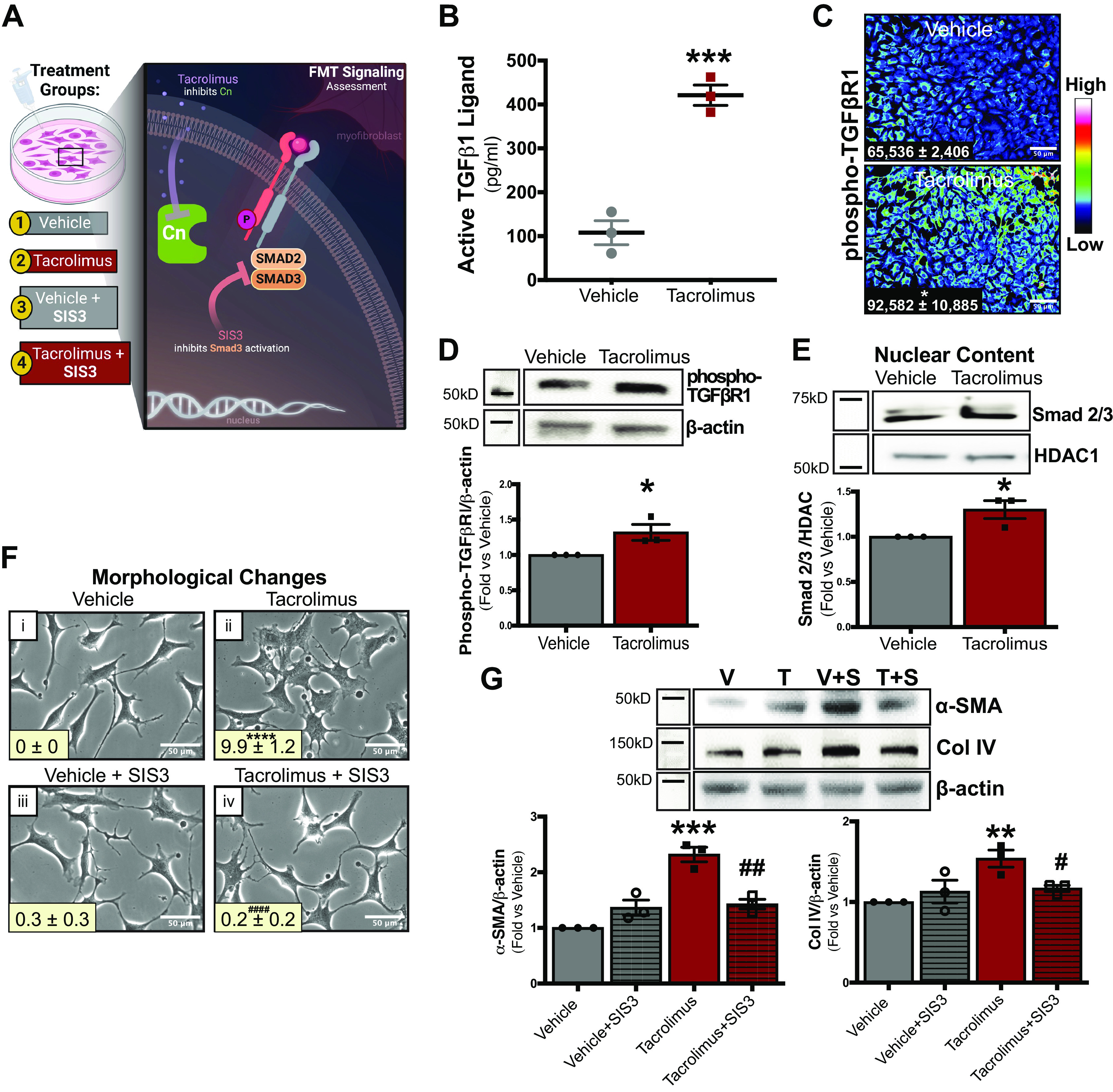
The transforming growth factor-β (TGF-β) signaling mediator Smad2/3 drives tacrolimus (T)-induced fibroblast-to-myofibroblast transition (FMT) and extracellular matrix production in renal fibroblasts. To examine the role of TGF-β receptor signaling in tacrolimus-induced FMT, renal fibroblasts were treated with either tacrolimus (1 nM) or vehicle (V; 0.01% ethanol) for 24 h (A). Type I TGF-β receptor subunit (TGFβRI) ligand secretion was quantified in media collected from both tacrolimus- and vehicle-treated cells by ELISA (B). TGFβRI activation was examined by visualizing phospho-TGFβRI expression via immunofluorescence (C) and quantified with CellProfiler software [expressed as arbitrary light units (alu)]. Western blot analysis (D) further quantified phospho-TGFβRI expression. Nuclear abundance of the TGF-β signaling mediator Smad2/3 was assessed by Western blot (E). To investigate the relevance of the TGF-β/Smad signaling pathway in tacrolimus-induced FMT and extracellular matrix accumulation, cellular morphological changes were visualized and quantified with ImageJ software in fibroblasts treated with either tacrolimus (1 nM) or vehicle (0.01% ethanol) in the presence or absence of the Smad3 inhibitor SIS3 (S; 500 nM; F). To confirm these morphological findings, α-smooth muscle actin (α-SMA) and collagen type IV (Col IV) abundance was assessed via Western blot (G). Representative images of at least 3 independent studies are shown. Values are means ± SE. Statistical tests were conducted using either two-way ANOVA or a t test to detect differences between the experimental groups. *P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.00005 vs. vehicle; #P < 0.05, ##P < 0.005, and ####P < 0.00005 vs. tacrolimus. Cn, calcineurin; HDAC, histone deacetylase 1.
