Abstract
The increasing prevalence of chronic kidney disease (CKD) is a major global public health concern. Despite the complicated pathogenesis of CKD, renal fibrosis represents the most common pathological condition, comprised of progressive accumulation of extracellular matrix in the diseased kidney. Over the last several decades, tremendous progress in understanding the mechanism of renal fibrosis has been achieved, and corresponding potential therapeutic strategies targeting fibrosis-related signaling pathways are emerging. Importantly, extracellular vesicles (EVs) contribute significantly to renal inflammation and fibrosis by mediating cellular communication. Increasing evidence suggests the potential of EV-based therapy in renal inflammation and fibrosis, which may represent a future direction for CKD therapy.
Keywords: Chronic kidney disease, Extracellular vesicles, Mechanism, Renal fibrosis, Therapy
Introduction
Due to the increased risk of end-stage renal disease and its devastating effects on the cardiovascular system, chronic kidney disease (CKD) is associated with high morbidity and mortality. There is a growing global burden of CKD, affecting 10% of adults worldwide; meanwhile, the global mortality rate attributed to CKD has increased by 41.5% in the last three decades [1,2]. Tubulointerstitial fibrosis, a defining feature of CKD, is characterized by extracellular matrix (ECM) accumulation and renal scarring, which lead to both structural and functional deterioration of the kidneys. The fibrogenesis process includes inflammatory cell infiltration, excessive fibroblast activation, overwhelming ECM deposition, tubular atrophy, and renal microvasculature rarefaction. Recent advances in single-cell RNA sequencing (scRNA-seq) have enabled tremendous progress in understanding the mechanisms behind renal fibrosis.
In past decades, clinically available pharmacological interventions for delaying CKD progression have been primarily restricted to renin-angiotensin-aldosterone system inhibitors. Transforming growth factor β (TGF-β) is the master regulator of fibrosis, and new agents that target the TGF-β signaling pathway are continually emerging. Particularly, there is mounting evidence supporting the critical role of extracellular vesicles (EVs) in renal physiology and pathology. EVs are considered key mediators of cellular communication participating in renal fibrosis progression. Importantly, EVs are promising therapeutic vectors due to their intrinsic contents and natural nanocarrier properties for small-molecule drugs as well as genetic therapies.
The purpose of this review is to provide new insights into the mechanisms of renal fibrosis, as well as prospective therapeutic approaches targeting pathological signaling and cellular events. The important role of EVs will be emphasized regarding the mechanisms and therapy of renal fibrosis.
Pathogenesis of renal fibrosis
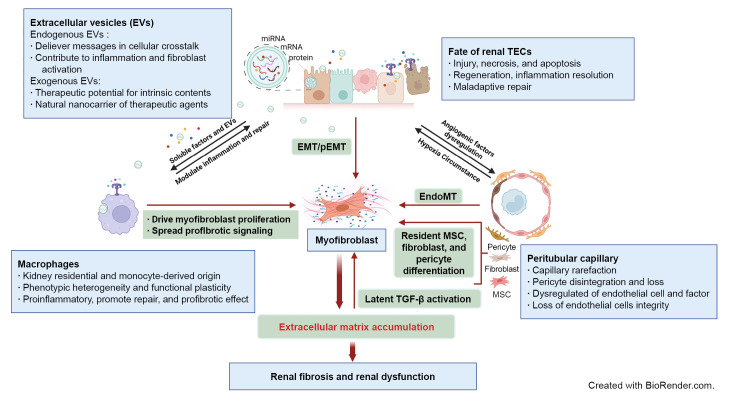
An overview of the complex interplays and critical events involved in renal fibrosis progression is shown in Fig. 1.
Figure 1. Schematic elucidation of cellular and signaling events in renal fibrosis.
Renal tubule injury acts as a driving force in fibrosis progression through communication with immune cells, peritubular capillary (PTC), and interstitial stroma cells via soluble or extracellular vesicle (EV) signaling. Persistent or severe injury leads to maladaptive repair of tubular epithelial cells (TECs) and subsequent EMT or pEMT, contributing to renal fibrosis. PTC rarefaction generates a hypoxic environment that promotes tubular atrophy. The phenotypic heterogeneity and functional plasticity elucidate the versatile roles of macrophages during inflammation, tissue repair, and fibrosis. Excessive accumulation of ECM components contributes to overactivation of myofibroblasts originating from multiple cellular sources and provides a substrate for latent transforming growth factor β (TGF-β) activation. Endogenous EVs play a notable role in delivery of messages in cellular communication, while exogenous EVs are being developed as new therapeutic agents for renal fibrosis.
AKI, acute kidney injury; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; EndoMT, endothelial-mesenchymal transition; MSC, mesenchymal stem cell; pEMT, partial epithelial-mesenchymal transition.
Maladaptive repair of tubule epithelial cells
Tubule epithelial cells (TECs) undergo adaptive and maladaptive repair after injury, which is crucial for determining whether kidney injury will be repaired or progress to CKD [3]. In most circumstances, however, residual inflammatory and fibrotic processes continue to propel disease progression despite recovery of renal function to baseline after acute injury [4]. In acute kidney injury (AKI), various damage-associated molecular patterns released from injured TECs interact with pattern recognition receptors on TECs, leading to production of proinflammatory cytokines and chemokines by the TECs and massive immune cell infiltration [5]. In turn, activated immune cells, especially macrophages, induce further TEC injury and necrosis [5,6]. Unresolved or excessive tubulointerstitial inflammation can lead to persistent kidney injury, which plays a central role in maladaptive repair of TECs.
A process of epithelial-mesenchymal transition (EMT) after injury has long been recognized and contributes to renal fibrosis as epithelial cells switch to mesenchymal cells [7]. However, whether epithelial cells undergo complete EMT and become matrix-producing cells depends on the condition of tissues and persistence of cytokine production [7]. Nevertheless, it has been demonstrated that dedifferentiated TECs remained adherent to the membrane and express markers of both epithelial and mesenchymal cells, a phenomenon called partial EMT (pEMT), contributing to renal fibrogenesis [8]. Importantly, injured and dedifferentiated proximal tubular cells are responsible for tissue repair other than fixed tubular progenitor cells, and proliferation of proximal tubules might be regulated by the EGFR-FOXM1 signaling pathway [9].
Recently, several scRNA-seq analyses further verified that maladaptive repair of TECs accelerates renal fibrosis. The scRNA-seq of a mouse AKI model identified a distinct proinflammatory and profibrotic role of failed-repair proximal tubule cells [10]. Another study found that maladaptive repair of proximal tubules could accelerate progressive interstitial fibrosis, which consequently promotes pericyte activation, peritubular capillary (PTC) loss, and matrix deposition [4]. In addition, new clusters of proximal tubular cells (present only following injury) with the ability to transfer pathological signaling to fibroblasts and macrophages were identified [11]. However, the underlying cellular and molecular mechanisms of maladaptive repair remain to be fully elucidated.
Peritubular capillary rarefaction
PTC rarefaction along with tubular atrophy is commonly detected in renal fibrosis. The level of PTC loss correlates with the severity of fibrosis [12]. Animal experiments have confirmed in CKD models such as the remnant kidney model [13] and unilateral ureteral obstruction (UUO) [14] that capillary density was negatively correlated with fibrosis. An antiangiogenic environment including deprivation of endothelial cell survival factors, upregulation of anti-angiogenic factors, dysfunction of endothelial cells, and loss of endothelial cell integrity contributes to the rarefaction of PTC [15]. In addition, pericyte disintegration and loss after kidney injury promoted instability of blood vessel structure and further capillary rarefaction. To date, the mechanism of PTC rarefaction is not clearly identified. However, inflammatory macrophages can block expression of tubular vascular endothelial growth factor A by infiltration and secretion of inflammatory cytokines, especially interleukin (IL) 1β and tumor necrosis factor α [16]. This blockage is regarded as a core event for PTC rarefaction. Additionally, as a key feature in ischemic kidney injury, the endothelial-to-mesenchymal transition (EndoMT) is depicted as the transition from typical endothelial cells to a profibrotic phenotype [17], which results in PTC rarefaction and CKD progression.
The versatile roles of macrophages
The phenotypic heterogeneity and functional plasticity elucidate the versatile roles of macrophages during tissue repair and fibrosis. Lineage tracing studies indicate that self-renewed kidney resident macrophages (KRMs) in adult kidneys largely originate from yolk sac erythro-myeloid progenitors (EMPs), fetal liver EMPs, and hematopoietic stem cells [18]. Once injury occurs, circulating monocytes from bone marrow infiltrate the kidney in inflammatory microenvironments [19]. A recent study demonstrated that KRMs and monocyte-derived infiltrated macrophages (IMs) play complementary functions in reducing tissue inflammation and fostering tissue repair [20].
Traditionally, IMs in kidney disease are grouped into either classically activated M1 macrophages associated with the TH1-like response or alternatively activated M2 macrophages that contribute to the TH2-like response. Specifically, M2 macrophages can be subdivided into three types based on diverse stimuli and functions [21]. Accumulation and activation of macrophages are directly related to kidney injury and fibrosis severity, and excessive profibrotic mediators secreted from M2 macrophages could drive myofibroblast proliferation and profibrotic signaling pathways [22]. Recent studies have illustrated that Ly6Chigh monocytes accumulate in the inflammatory kidney and differentiate into three subpopulations, including the proinflammatory CD11b+/Ly6Chigh population presented at the onset of renal injury, the CD11b+/Ly6Cint population dominant in the renal repair phase, and the profibrotic CD11b+/Ly6Clow population that emerges in renal fibrosis [23].
In scRNA-seq analysis, macrophage diversity can be deciphered unbiasedly or macrophage clusters can be explored according to phenotype and cell function, showing the complexity of macrophages [24]. For example, recent scRNA-seq research identified a unique population of S100A9hiLy6Chi IMs mediating the initiation and amplification of inflammation in AKI, and blockade of S100a8/a9 signaling exhibited renal protective effects in an ischemia-reperfusion injury (IRI) model [20]. Therefore, a precise understanding of the dynamics and functional characteristics of macrophages under different microenvironments could offer specific therapeutic targets for kidney diseases.
Activation of matrix-producing cells
Excessive ECM accumulation is the key characteristic of renal fibrosis, and studies have been conducted to define the cellular sources contributing to pathological deposition of ECM. Myofibroblasts are commonly regarded as the predominant matrix-producing cell in diseased kidneys [25]. Renal resident fibroblasts can transdifferentiate into myofibroblasts with reduced production of fibroblast-derived erythropoietin, leading to renal anemia and consequent CKD progression [26].
Traditionally, α-smooth muscle actin is considered the marker for myofibroblasts, while a recent scRNA-seq study proposed Postn as another identifier for myofibroblasts with high ECM production [27]. Interestingly, there is high heterogeneity of myofibroblasts in terms of cell origin and function, similar to diverse macrophages in diseased kidneys. scRNA-seq applied in human kidney fibrosis revealed that myofibroblasts mainly originate from diverse resident mesenchymal cells, primarily distinct fibroblast and pericyte populations, far more than from fibrocytes [27]. Mesenchymal stem cells (MSCs) were previously proposed as contributors of myofibroblasts [28], and new evidence suggested that Gli1+ MSC-like cells represent a myofibroblast pool in response to injury and contribute to fibrosis development [29,30]. Although EMT and EndoMT are mechanisms involved in renal fibrosis, myofibroblasts from transdifferentiated renal tubular cells or endothelial cells have been reported to account for only a small fraction [27]. Overall, myofibroblasts are responsible for excessive ECM synthesis and deposition, and further research is needed to clarify the full map of matrix-producing cells during fibrosis development.
Myofibroblasts produce collagen fibers when activated, resulting in excessive ECM deposition. The remodeling of ECM is in an equilibrium process. The ongoing ECM protein synthesis and degradation are orchestrated by matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs), both of which are considered key enzymes responsible for remodeling of ECM. Thus, dysregulation of MMP/TIMP activity is associated with progression of renal fibrosis [31]. Interestingly, the mechanical structure of ECM is not simply a scaffold, but rather a substrate to bind growth factors, particularly latent TGF-β1. ECM is capable of activating TGF-β1 by supplying the necessary mechanical resistance. The latent TGF-β–binding protein (LTBP) covalently binds the latency-associated peptide (LAP) together with TGF-β to form the large latent complex. LTBPs interact with ECM components and localize latent TGF-β in the ECM. When integrins on the cell surface attach to the Arg-Gly-Asp (RGD) binding site of LAP, TGF-β1 is released and activated through tension generated between integrins and ECM [32]. Moreover, activation of latent TGF-β is enhanced as ECM stiffness increases, leading to increased TGF-β signaling [33,34].
Inflammation and fibrosis signaling activated by extracellular vesicles
EVs are small membrane vesicles of two major subtypes: exosomes (40 to 160 nm), which originate from endosomes, and ectosomes (50 nm to 1 μm), which are derived from direct plasma membrane budding [35]. Increasing evidence supports the idea that EVs selectively transfer specific signals to regulate organ development, immune responses, and disease. Therefore, understanding the signals transferred by EVs may help shed light on the mechanisms of renal fibrosis.
As the primary component of the tubulointerstitium, TECs are particularly vulnerable to injury, which accelerates renal disease progression. The secreted proinflammatory mediators then guide inflammatory cells, including monocytes/macrophages, dendritic cells, neutrophils, lymphocytes, and mast cells, to the injured sites to provoke inflammation and cell death [6,36]. Recent studies support a notable role of EVs in renal inflammation via mediation of tubular-macrophage crosstalk. After injury, TECs increase the secretion of EVs carrying proinflammatory-related cargoes, such as CC-chemokine ligand 2 (CCL2) messenger RNA (mRNA) and functional microRNA (miRNAs: miRNA-23a, miRNA-19b-3p, etc.), which are transferred to initiate macrophage activation and migration and augment tubulointerstitial inflammation [37–39]. In addition, EVs are essential signal messengers in the proximal-to-distal tubular communication in pathological conditions [40]. Moreover, EVs could also participate in renal fibrosis via communication with interstitial fibroblasts. Injured TEC-derived exosomes enriched with TGF-β1 mRNA promote fibroblast activation [41]. Furthermore, increasing reports suggest that EVs containing various miRNAs (miR-196b-5p, miR-150, and miR-21) can activate fibroblasts and intensify renal fibrosis [42–44]. Recently, tubular cell-derived exosomal osteopontin was identified as responsible for activation of fibroblasts and promotion of renal fibrosis development [45].
Therefore, EVs released from injured renal cells are loaded with signal molecules of inflammation and fibrosis, which favor amplification of unresolved and prolonged inflammatory proteins and further serve as a crucial trigger of tissue fibrogenesis.
Therapy of renal fibrosis
Emerging transforming growth factor-β–targeted treatment
Fibrosis is the ultimate common pathway for CKD in spite of the underlying etiology, and antifibrotic agents are crucial for treatment of CKD. Here, we mainly discuss emerging therapeutic options targeting TGF-β in renal fibrosis and CKD.
TGF-β is linked with fibrosis of various organs. Previous evidence demonstrated that TGF-β participates in pathological fibrosis processes, including mediating ECM dysregulation, transdifferentiation of intrinsic cells, and mesangial cell proliferation. Therefore, TGF-β signaling represents a critical target for renal fibrosis.
Pirfenidone is a small synthetic inhibitor that blocks the TGF-β promotor and has antifibrotic and anti-inflammatory properties. It has been widely used for idiopathic pulmonary fibrosis treatment in clinical studies [46]. In many animal models of renal disease, pirfenidone also exerts similar effects [47], however, its potential in clinical settings remains to be investigated. The ongoing TOP-CKD trial (NCT04258397), the largest pirfenidone phase II study enrolling 200 participants, is estimated to be completed by December 2024. Similarly, pentoxifylline, a clinically available drug, was reported to downregulate TGF-β1 expression, delay progression of CKD, and reduce cardiovascular risk [48].
Compared to TGF-β deficiency probably causing severe immune dysregulation, antibody neutralization of TGF-β is recognized to have higher security with fewer adverse effects. Multiple preclinical investigations have revealed that direct TGF-β neutralization could halt the development of fibrosis. Fresolimumab and LY2382770, human monoclonal antibodies that neutralize TGF-β1, have been evaluated in phase Ⅱ trials in patients with steroid-resistant focal segmental glomerulosclerosis (FSGS) and diabetic nephropathy, respectively [49,50]. Unfortunately, neither achieved the expected clinical outcome, which may need to be confirmed by larger and more robust studies. Since integrin αvβ6 can activate latent TGF-β, targeted integrin αvβ6 blockade by antibodies or small molecules offers an option for inhibition of TGF-β-induced fibrosis. The monoclonal antibody STX-100 (BG00011), which specifically blocks integrin αvβ6, has shown potential as an antifibrotic medication [51]. A phase II study with STX-100 (NCT00878761) administered to individuals with chronic allograft dysfunction, however, resulted in discontinuation for unknown reasons.
To avoid adverse events caused by a complete blockade of TGF-β, selective blockers of the TGF-β downstream signaling pathway have attracted increasing attention. Further basic research and clinical trials are required to discover a precise approach to TGF-β inhibition.
Potential application of extracellular vesicles in renal fibrosis therapy
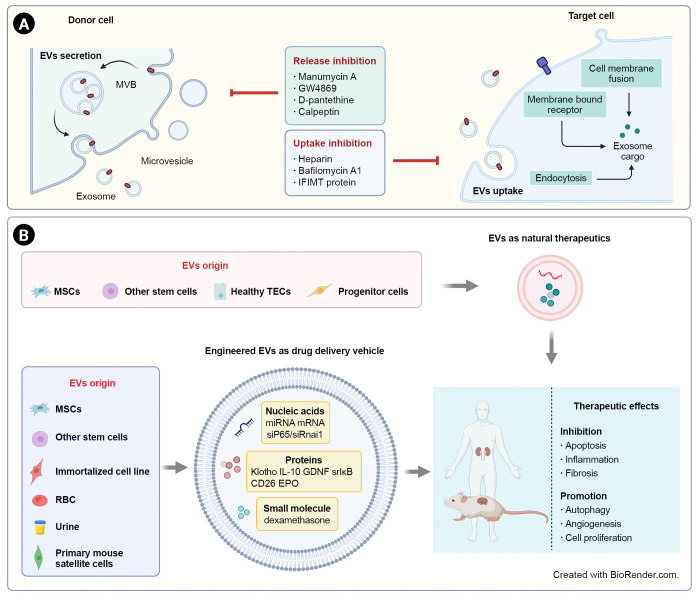
Increasing evidence suggests that pathological signaling delivered by EVs could be essential for tubulointerstitial communication in renal inflammation and fibrosis [38]. Therefore, inhibition of endogenous damage-associated EVs is a potential therapeutic strategy. Moreover, the therapeutic functions of MSC-derived EVs have given rise to increasing interest due to their intrinsic contents. Notably, EVs could also act as a natural “Trojan horse” to deliver a variety of drugs to treat kidney diseases. Schematic EV-based therapeutic strategies for renal fibrosis are shown in Fig. 2.
Figure 2. EV-based therapeutic strategies for renal fibrosis.
(A) Due to the pathological effects of EVs in renal inflammation and fibrosis, inhibition of EV secretion or uptake is a potential strategy for kidney diseases. (B) EVs derived from stem cells or healthy renal intrinsic cells could act as direct natural therapeutics. EVs can also be used as delivery vehicles for a variety of drugs, including nucleic acids, proteins, and small molecules. EV-based treatments have shown therapeutic effects on renal fibrosis through inhibition of apoptosis, inflammation, and fibrosis and promotion of autophagy, angiogenesis, and proliferation.
EVs, extracellular vesicles; EPO, erythropoietin; GDNF, glial-derived neurotrophic factor; IL, interleukin; miRNA, microRNA; mRNA, messenger RNA; MSCs, mesenchymal stem cells; MVB, multivesicular body; RBC, red blood cell; srIκB, super-repressor IκB; TECs, tubular epithelial cells.
Inhibition of pathogenic extracellular vesicles
EVs are secreted by parent cells and travel to neighboring or remote sites to exert their function, a process that could be inhibited by targeting the release and uptake of EVs. Pharmacologically, a number of agents have been demonstrated to inhibit EV secretion through different mechanisms. Manumycin A, the most widely used farnesyltransferase inhibitor, is necessary for exosome synthesis in the endosomal sorting complex required for transport (ESCRT)-dependent pathway and has been shown in an in vitro model to block exosome release to support the repair of damaged renal epithelium [52,53]. GW4869 is a blockade of neutral sphingomyelinase (nSMase), which mediates ESCRT-independent intraluminal vesicle formation, and has been widely reported as a pharmacological agent to inhibit exosome release in various cancers as well as kidney diseases. Reduced exosome secretion after GW4869 treatment inhibited fibroblast activation and ECM deposition in unilateral IRI in mice [54]. Phosphatidylserine externalization plays a crucial role in membrane budding and formation of microvesicles (MVs). The pantothenic acid derivative pantethine was demonstrated to impair MV release by preventing the transfer of phosphatidylserine. In an experimental model, mice treated orally with D-pantethine showed alleviation of fibrosis, and such a protective function might be associated with reduction of endothelial-derived MVs in circulation [55]. Calpain could be activated by calcium to regulate cytoskeleton remodeling and then increase MV release. As a calpain inhibitor, calpeptin treatment could reduce bleomycin-induced pulmonary fibrosis by inhibiting EMT-related markers and the TGF-β1 signaling pathway, which might be related to MV reduction [56].
EVs are significant intercellular communication mediators that interact with recipient cells in an autocrine, paracrine, or endocrine manner [57] through three mechanisms: membrane fusion, receptor (direct) interaction, and internalization [58]. Hence, they may provide an alternative method to inhibit exosome function by blocking uptake. Heparan sulfate proteoglycans (HSPGs) participate in the internalization of cancer cell-derived exosomes, which depend on intact HSPG synthesis and HS sulfation in target cells. Heparin as an HS mimetic inhibits exosome uptake dose dependently [59]. In addition, Bonsergent et al. [60] found that EV uptake is a slow process by quantification analysis, and content delivery can be inhibited by bafilomycin A1 and IFIMT protein overexpression in a pH-dependent manner.
Due to the heterogeneity of EVs and recipient cells, further investigation is warranted to clarify the precise mechanism of formation and uptake of EVs for developing precise therapeutic strategies targeting pathogenic EVs.
Utilization of mesenchymal stem cell-extracellular vesicles as therapeutic agents
Remarkable therapeutic effects of anti-inflammation, antifibrosis, and proregeneration have been demonstrated by MSCs for treatment of kidney disease. However, there are safety issues related to immune responses, toxicity, and carcinogenicity [61]. MSCs function in a paracrine or endocrine manner and are coordinated by secretomes, growth factors, cytokines, and EVs [62]. Compared to MSCs, EVs derived from MSCs are characterized by higher safety, lower immunogenicity, easier preservation, and genetic stability.
A growing number of studies are exploring the potential properties of MSC-EVs in CKD models. Intravenous administration of EVs derived from bone marrow MSCs (BM-MSC) ameliorated tubular necrosis and interstitial fibrosis and improved renal function in a mouse model of aristolochic acid-induced nephropathy [63]. EVs originated from BM-MSCs and human liver stem-like cells also attenuated fibrosis in diabetic nephropathy due to modulation of fibrosis-related gene expression by miRNA cargoes [64]. In a clinical pilot study involving 40 CKD patients with eGFR between 15 and 60 mL/min/1.73 m2, both intravenous and intraarterial administration of cell-free MSC-EVs from umbilical cord blood regulated immune response and improved renal function [65]. EVs containing miR-26a-5p from adipose-derived MSCs protected against diabetic nephropathy by targeting toll-like receptor 4 [66]. Additionally, exosomes of human umbilical cord MSCs have been shown to promote nuclear YAP shuttle to cytoplasm and to reduce matrix accumulation by transporting casein kinase 1 δ and β-TRCP to target cells [67]. Interestingly, human urine-derived stem cell exosomes protected against diabetic nephropathy by reducing apoptosis of podocytes and enhancing angiogenesis and cell survival [68].
In conclusion, MSC-EVs may become an alternative therapeutic tool for kidney disease, and further research should be conducted to promote the transition of MSC-EVs to clinical application.
Extracellular vesicles as therapeutic delivery vehicles
As natural membrane structures, EV capacity to transfer biomolecules to recipient cells has attracted considerable attention for its potential to overcome limitations of liposomes and other synthetic drug delivery systems. EV-based therapies offer many outstanding advantages due to the natural lipid structure and modifiable membrane properties achieved through manipulation of parent cells to improve stability as well as by targeting specific tissues and cells [69].
Endogenous and exogenous loading are two principal approaches to integrate therapeutic drugs into exosomes [70]. The technical methodology can be found in another published review [71]. Here, we summarize the studies regarding application of EVs as a therapeutic agent delivery system in renal disease (Table 1) [72–90].
Table 1.
Therapeutic applications of EVs in kidney disease
| Functional agent | EV origin | Animal model | Efficacy | Reference |
|---|---|---|---|---|
| Nucleic acid | ||||
| miR-34c-5p | BM-MSC | UUO | Reduced pericyte, fibroblast, and macrophage activation and renal fibrosis | [74] |
| I/R | ||||
| miR-186-5p agomir | MSC | UUO | Inhibited ECM accumulation and EMT process | [76] |
| miR-125a | AD-MSC | DN | Reduced mesangial hyperplasia, expansion of mesangial matrix, and kidney fibrosis | [77] |
| miR-26a-5p | HEK293 cell | UUO | Suppressed muscle wasting and renal fibrosis by targeting FoxO1 and CTGF | [79] |
| let-7i-5p antagomir | BM-MSC | UUO | Reduced renal fibrosis by activating the TSC1/ mTOR pathway | [80] |
| miR-16-5p | Urine-derived stem cells | DN | Improved diabetic nephropathy and inhibited podocyte apoptosis by reducing VEGF-A | [81] |
| miR-29 | Primary mouse satellite cell | UUO | Ameliorated skeletal muscle atrophy and attenuated kidney fibrosis | [82] |
| miR-20b-3p | AD-MSC | Ethylene glycol-induced hyperoxaluria | Reduced cell autophagy and inflammatory responses | [83] |
| miR-let7c | BM-MSC | UUO | Attenuated kidney injury and reduced ECM accumulation and fibrotic-related gene expression | [84] |
| siP65 and siSnai1 | Red blood cell | I/R-induced AKI or UUO | Alleviated tubulointerstitial inflammation and fibrosis and abrogated the transition to CKD | [75] |
| Oct-4 mRNA | UC-MSC | I/R | Increased the therapeutic effects of | [85] |
| MSC-EVs to attenuate kidney fibrosis | ||||
| Protein | ||||
| Klotho | Urine/fibroblast | AKI generated by glycerol injection | Accelerated renal recovery, stimulated tubular cell proliferation, and reduced inflammation; reduced renal retention and tissue injury; promoted amelioration of renal function | [86] |
| IL-10 protein | RAW264.7 cell | I/R-induced AKI | Ameliorated renal tubular injury and inflammation and prevented AKI-to-CKD transition | [73] |
| GDNF | AD-MSC | UUO | Ameliorated peritubular capillary loss in tubulointerstitial fibrosis | [87] |
| Super-repressor IκBα | HEK293T cell | CLP-induced sepsis | Attenuated mortality, acute organ injury, and inflammation by inhibiting the NF-κB pathway | [88] |
| Super-repressor IκBα | HEK293T cell | I/R-induced AKI | Alleviated renal damage and ameliorated inflammation and apoptosis | [89] |
| CD26 | TCMK1 cell | I/R-induced AKI | Protected against kidney injury by maintaining proliferation and dissipating inflammation | [90] |
| Erythropoietin | Kidney MSC | Model of CKD and renal anemia | Improved hemoglobin levels and renal function in CKD mice and exerted antifibrotic and anti-inflammatory effects | [91] |
| Small molecule | ||||
| Dexamethasone | RAW264.7 cell | LPS- or ADR-induced nephropathy | Suppressed renal inflammation and fibrosis without apparent glucocorticoid adverse effects | [72] |
AD, adipose mesenchymal stem cell; ADR, adriamycin; AKI, acute kidney injury; BM, bone marrow; CKD, chronic kidney disease; CLP, cecal ligation and puncture; CTGF, connective tissue growth factor; DN, diabetic nephropathy; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; EV, extracellular vesicle; GDNF, glial-derived neurotrophic factor; I/R, ischemia/reperfusion; LPS, lipopolysaccharide; mRNA, messenger RNA; MSC, mesenchymal stem cell; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κB; UC, umbilical cord; UUO, unilateral ureteral obstruction; VEGF-A, vascular endothelial growth factor A.
Molecular drugs
EVs have been extensively used as delivery vectors for small molecules in recent studies. Curcumin carried by exosomes had excellent biological function in terms of solubility, stability, and bioavailability. Curcumin encapsulated by exosomes exerted anti-inflammatory effects in lipopolysaccharide-induced brain inflammation and myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis [91]. Additionally, exosome-loaded doxorubicin showed less accumulation in off-target tissues and was less cardiotoxic than unmodified doxorubicin [92,93]. Dexamethasone-loaded macrophage-derived MVs exhibited superior anti-inflammatory and antifibrotic activity without apparent glucocorticoid adverse effects [72], suggesting the possibility of EVs for drug transfer in renal disease.
Therapeutic proteins
Exosomes have an intrinsic capacity to cross biological barriers. Macrophage-derived exosomes without modification can penetrate the blood-brain barrier to transfer brain-derived neurotrophic factor to the central nervous system [94]. Recently, we successfully constructed IL-10-loaded EVs by engineering macrophages to target the injured kidney, which significantly improved renal tubular injury and inflammation and prevented the transition to CKD [73]. Concerning the stability and validity of large molecular cargoes, however, there are technical obstacles to efficiently load proteins into EVs. Increasing attempts had been made to solve this problem; for example, Leidal et al. [95] successfully loaded RNA-binding proteins into EVs via LC3-conjugation machinery.
Genetic materials
Natural EVs can carry both coding RNAs (mRNAs) and noncoding RNAs (long noncoding RNAs, miRNAs, and circular RNAs), which suggests outstanding ability in transferring diverse RNAs for therapeutic purposes [96]. Particularly, miRNAs in engineered EVs have been widely studied in kidney diseases. For example, BM-MSC-derived exosomes inhibited core fucosylation by delivering miR-34c-5p to reduce activation of pericytes, fibroblasts, macrophages, and renal interstitial fibrosis [74]. Furthermore, mRNAs loaded in EVs could be applied for personalized tumor vaccines [97] and the coronavirus disease 2019 pandemic [98]. In addition, EVs loaded with small interfering RNA (siRNA) could also be a promising strategy to mediate gene silencing for cancer therapy [99]. Engineered red blood cell-derived EVs modified with peptide targeting KIM-1 have been constructed and successfully delivered siRNAs against P65 and Snai1 into injured kidneys. Dual inhibition of P65 and Snai1 expression significantly alleviated kidney inflammation and fibrosis in mouse models of IRI and UUO [75].
Conclusion and perspectives
In recent years, it has been shown that maladaptive repair of TECs, PTC rarefaction, activation and proliferation of myofibroblasts, diverse functions of macrophages, ECM hemostasis, and EV-mediated cellular communications are important in tubulointerstitial inflammation and fibrosis. However, the accurate mechanism of renal fibrosis remains to be fully clarified. By combination and integration of single-cell and multiomics techniques, it is now possible to better understand disease mechanisms [100–102]. In addition, EV-mediated cellular communication also provides a new insight into the pathogenesis process of renal fibrosis.
Despite the development of therapeutic agents ranging from chemical compounds to gene therapies against renal fibrosis, clinical translation from bench to bedside is often limited due to the slow progression of disease and heterogeneity of patients as well as lack of noninvasive biomarkers for renal fibrosis [103]. Recent research showed that positron emission tomography imaging of collagen and molecular imaging of fibrosis may allow efficient, noninvasive, quantitative, and longitudinal results [104]. Urinary EVs released from intrinsic renal cells hold the potential to predict and monitor CKD progression as a “fluid biopsy” approach; for instance, miR-29 in urine EVs has been identified as a biomarker of renal fibrosis [105].
EV-based treatments are promising approaches to realize targeted therapy. However, clinical application of EVs in kidney disease is far from practical. Low production of EVs greatly hinders their clinical application, which encourages advances in stimulating EV shedding and production, but the properties and functions of EVs should be evaluated further. In addition, engineered EVs, including EVs from genetically engineered cells, post-modified EVs (drug loaded, surface modified), and EV-inspired liposomes have been developed to enhance therapeutic activity [106]. MSC-EVs and EVs from other sources have been tested for safety and efficacy in numerous ongoing clinical trials for diseases including diabetes, SARS-CoV-2 pneumonia, Alzheimer’s disease, and various tumors [106]. Due to the heterogeneity of EVs, it is urgent to establish a good unified standard to achieve large-scale and efficient production of clinical-grade EVs for clinical application. Therapeutic EV production is a complex process in which minor changes can have significant impacts on product quality and efficacy [106]. Furthermore, administration route, dosage, and biological distribution of EVs in vivo should be considered before EV products are applied in patients. The underlying mechanisms of EV production, secretion, and uptake are not fully clarified at present. Robust studies of basic EV biology are needed to enable clinical translation. Emerging advanced technologies such as super resolution microscopy, single extracellular vesicle assay, and nanoflow cytometry could be useful tools to achieve deep understanding of EV biology [107].
Overall, with progress in understanding the mechanisms of renal fibrosis as well as the emerging therapeutic strategies, particularly EV-based therapies, we look forward to a new era of precise and targeted treatments for renal fibrosis.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This study is supported by grants from the National Natural Scientific Foundation of China (grant No. 82122011, 81970616, and 82241045).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: CW, SWL, LLL
Funding acquisition, Supervision: BCL, LLL
Writing–original draft: CW, SWL
Writing–review & editing: All authors
All authors read and approved the final manuscript.
References
- 1.Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398:786–802. doi: 10.1016/S0140-6736(21)00519-5. [DOI] [PubMed] [Google Scholar]
- 2.GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu SM, Bonventre JV. Acute kidney injury and maladaptive tubular repair leading to renal fibrosis. Curr Opin Nephrol Hypertens. 2020;29:310–318. doi: 10.1097/MNH.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu BC, Tang TT, Lv LL, Lan HY. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93:568–579. doi: 10.1016/j.kint.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Mulay SR, Linkermann A, Anders HJ. Necroinflammation in kidney disease. J Am Soc Nephrol. 2016;27:27–39. doi: 10.1681/ASN.2015040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 8.Sheng L, Zhuang S. New insights into the role and mechanism of partial epithelial-mesenchymal transition in kidney fibrosis. Front Physiol. 2020;11:569322. doi: 10.3389/fphys.2020.569322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang-Panesso M, Kadyrov FF, Lalli M, et al. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J Clin Invest. 2019;129:5501–5517. doi: 10.1172/JCI125519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A. 2020;117:15874–15883. doi: 10.1073/pnas.2005477117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu YA, Liao CT, Raybould R, et al. Single-nucleus RNA sequencing identifies new classes of proximal tubular epithelial cells in kidney fibrosis. J Am Soc Nephrol. 2021;32:2501–2516. doi: 10.1681/ASN.2020081143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi YJ, Chakraborty S, Nguyen V, et al. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol. 2000;31:1491–1497. doi: 10.1053/hupa.2000.20373. [DOI] [PubMed] [Google Scholar]
- 13.Kang DH, Joly AH, Oh SW, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi I, Tchao BN, Burger ML, et al. Vascular endothelial cadherin modulates renal interstitial fibrosis. Nephron Exp Nephrol. 2012;120:e20. doi: 10.1159/000332026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kida Y. Peritubular capillary rarefaction: an underappreciated regulator of CKD progression. Int J Mol Sci. 2020;21:8255. doi: 10.3390/ijms21218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koller GM, Schafer C, Kemp SS, et al. Proinflammatory mediators, IL (interleukin)-1β, TNF (tumor necrosis factor) α, and thrombin directly induce capillary tube regression. Arterioscler Thromb Vasc Biol. 2020;40:365–377. doi: 10.1161/ATVBAHA.119.313536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jourde-Chiche N, Fakhouri F, Dou L, et al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 2019;15:87–108. doi: 10.1038/s41581-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Hirschi KK. Tissue-resident macrophage development and function. Front Cell Dev Biol. 2021;8:617879. doi: 10.3389/fcell.2020.617879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15:144–158. doi: 10.1038/s41581-019-0110-2. [DOI] [PubMed] [Google Scholar]
- 20.Yao W, Chen Y, Li Z, et al. Single cell RNA sequencing identifies a unique inflammatory macrophage subset as a druggable target for alleviating acute kidney injury. Adv Sci (Weinh) 2022;9:e2103675. doi: 10.1002/advs.202103675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T, Cao Q, Wang Y, Harris DC. M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int. 2019;95:760–773. doi: 10.1016/j.kint.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Chen J, Xu J, Xie J, Harris DC, Zheng G. The role of macrophages in kidney fibrosis. Front Physiol. 2021;12:705838. doi: 10.3389/fphys.2021.705838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clements M, Gershenovich M, Chaber C, et al. Differential Ly6C expression after renal ischemia-reperfusion identifies unique macrophage populations. J Am Soc Nephrol. 2016;27:159–170. doi: 10.1681/ASN.2014111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway BR, O’Sullivan ED, Cairns C, et al. Kidney single-cell atlas reveals myeloid heterogeneity in progression and regression of kidney disease. J Am Soc Nephrol. 2020;31:2833–2854. doi: 10.1681/ASN.2020060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramann R, Dirocco DP, Maarouf OH, Humphreys BD. Matrix producing cells in chronic kidney disease: origin, regulation, and activation. Curr Pathobiol Rep. 2013;1:301–311. doi: 10.1007/s40139-013-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asada N, Takase M, Nakamura J, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121:3981–3990. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuppe C, Ibrahim MM, Kranz J, et al. Decoding myofibroblast origins in human kidney fibrosis. Nature. 2021;589:281–286. doi: 10.1038/s41586-020-2941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson S, Trial J, Soeller C, Entman ML. Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction. Cardiovasc Res. 2011;91:99–107. doi: 10.1093/cvr/cvr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramann R, Schneider RK, DiRocco DP, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider RK, Mullally A, Dugourd A, et al. Gli1+ mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell. 2017;20:785–800.e8. doi: 10.1016/j.stem.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakiyanov O, Kalousová M, Zima T, Tesař V. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in kidney disease. Adv Clin Chem. 2021;105:141–212. doi: 10.1016/bs.acc.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Shi M, Zhu J, Wang R, et al. Latent TGF-β structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian B, Ding X, Song Y, et al. Matrix stiffness regulates SMC functions via TGF-β signaling pathway. Biomaterials. 2019;221:119407. doi: 10.1016/j.biomaterials.2019.119407. [DOI] [PubMed] [Google Scholar]
- 35.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen PS, Li YP, Ni HF. Morphology and evaluation of renal fibrosis. Adv Exp Med Biol. 2019;1165:17–36. doi: 10.1007/978-981-13-8871-2_2. [DOI] [PubMed] [Google Scholar]
- 37.Li ZL, Lv LL, Tang TT, et al. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95:388–404. doi: 10.1016/j.kint.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Lv LL, Feng Y, Wen Y, et al. Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol. 2018;29:919–935. doi: 10.1681/ASN.2017050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv LL, Feng Y, Wu M, et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020;27:210–226. doi: 10.1038/s41418-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gildea JJ, Seaton JE, Victor KG, et al. Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin Biochem. 2014;47:89–94. doi: 10.1016/j.clinbiochem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borges FT, Melo SA, Özdemir BC, et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu R, Li X, Peng C, et al. miR-196b-5p-enriched extracellular vesicles from tubular epithelial cells mediated aldosterone-induced renal fibrosis in mice with diabetes. BMJ Open Diabetes Res Care. 2020;8:e001101. doi: 10.1136/bmjdrc-2019-001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan H, Peng R, Mao L, Fang F, Xu B, Chen M. Injured tubular epithelial cells activate fibroblasts to promote kidney fibrosis through miR-150-containing exosomes. Exp Cell Res. 2020;392:112007. doi: 10.1016/j.yexcr.2020.112007. [DOI] [PubMed] [Google Scholar]
- 44.Zhao S, Li W, Yu W, et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics. 2021;11:8660–8673. doi: 10.7150/thno.62820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S, Zhang M, Li J, et al. β-catenin-controlled tubular cell-derived exosomes play a key role in fibroblast activation via the OPN-CD44 axis. J Extracell Vesicles. 2022;11:e12203. doi: 10.1002/jev2.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan SD, Costabel U, Albera C, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment. Respir Med. 2019;153:44–51. doi: 10.1016/j.rmed.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 47.Bai X, Nie P, Lou Y, et al. Pirfenidone is a renal protective drug: mechanisms, signalling pathways, and preclinical evidence. Eur J Pharmacol. 2021;911:174503. doi: 10.1016/j.ejphar.2021.174503. [DOI] [PubMed] [Google Scholar]
- 48.de Morales AM, Goicoechea M, Verde E, et al. Pentoxifylline, progression of chronic kidney disease (CKD) and cardiovascular mortality: long-term follow-up of a randomized clinical trial. J Nephrol. 2019;32:581–587. doi: 10.1007/s40620-019-00607-0. [DOI] [PubMed] [Google Scholar]
- 49.Vincenti F, Fervenza FC, Campbell KN, et al. A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int Rep. 2017;2:800–810. doi: 10.1016/j.ekir.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klinkhammer BM, Goldschmeding R, Floege J, Boor P. Treatment of renal fibrosis-turning challenges into opportunities. Adv Chronic Kidney Dis. 2017;24:117–129. doi: 10.1053/j.ackd.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Tampe D, Zeisberg M. Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol. 2014;10:226–237. doi: 10.1038/nrneph.2014.14. [DOI] [PubMed] [Google Scholar]
- 52.Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. 2019;9:1703244. doi: 10.1080/20013078.2019.1703244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, Zhang W, Yao Q, et al. Exosome production and its regulation of EGFR during wound healing in renal tubular cells. Am J Physiol Renal Physiol. 2017;312:F963–F970. doi: 10.1152/ajprenal.00078.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X, Zhao S, Li W, et al. Tubular cell-derived exosomal miR-150-5p contributes to renal fibrosis following unilateral ischemia-reperfusion injury by activating fibroblast in vitro and in vivo. Int J Biol Sci. 2021;17:4021–4033. doi: 10.7150/ijbs.62478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kavian N, Marut W, Servettaz A, et al. Pantethine prevents murine systemic sclerosis through the inhibition of microparticle shedding. Arthritis Rheumatol. 2015;67:1881–1890. doi: 10.1002/art.39121. [DOI] [PubMed] [Google Scholar]
- 56.Tabata C, Tabata R, Nakano T. The calpain inhibitor calpeptin prevents bleomycin-induced pulmonary fibrosis in mice. Clin Exp Immunol. 2010;162:560–567. doi: 10.1111/j.1365-2249.2010.04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Niel G, Carter DR, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23:369–382. doi: 10.1038/s41580-022-00460-3. [DOI] [PubMed] [Google Scholar]
- 58.Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–543. doi: 10.1016/j.jconrel.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 59.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Théry C, Lavieu G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun. 2021;12:1864. doi: 10.1038/s41467-021-22126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lelek J, Zuba-Surma EK. Perspectives for future use of extracellular vesicles from umbilical cord- and adipose tissue-derived mesenchymal stem/stromal cells in regenerative therapies: synthetic review. Int J Mol Sci. 2020;21:799. doi: 10.3390/ijms21030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(Suppl 2):789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 63.Kholia S, Herrera Sanchez MB, Cedrino M, et al. Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front Cell Dev Biol. 2020;8:188. doi: 10.3389/fcell.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grange C, Tritta S, Tapparo M, et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. 2019;9:4468. doi: 10.1038/s41598-019-41100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nassar W, El-Ansary M, Sabry D, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duan Y, Luo Q, Wang Y, et al. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J Biol Chem. 2020;295:12868–12884. doi: 10.1074/jbc.RA120.012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji C, Zhang J, Zhu Y, et al. Exosomes derived from hucMSC attenuate renal fibrosis through CK1δ/β-TRCP-mediated YAP degradation. Cell Death Dis. 2020;11:327. doi: 10.1038/s41419-020-2510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang ZZ, Liu YM, Niu X, et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24. doi: 10.1186/s13287-016-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu P, Zhang B, Ocansey DK, Xu W, Qian H. Extracellular vesicles: a bright star of nanomedicine. Biomaterials. 2021;269:120467. doi: 10.1016/j.biomaterials.2020.120467. [DOI] [PubMed] [Google Scholar]
- 71.Tang TT, Wang B, Lv LL, Dong Z, Liu BC. Extracellular vesicles for renal therapeutics: state of the art and future perspective. J Control Release. 2022;349:32–50. doi: 10.1016/j.jconrel.2022.06.049. [DOI] [PubMed] [Google Scholar]
- 72.Tang TT, Lv LL, Wang B, et al. Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: an efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics. 2019;9:4740–4755. doi: 10.7150/thno.33520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang TT, Wang B, Wu M, et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci Adv. 2020;6:eaaz0748. doi: 10.1126/sciadv.aaz0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu X, Shen N, Liu A, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-34c-5p ameliorates RIF by inhibiting the core fucosylation of multiple proteins. Mol Ther. 2022;30:763–781. doi: 10.1016/j.ymthe.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang TT, Wang B, Li ZL, et al. Kim-1 targeted extracellular vesicles: a new therapeutic platform for RNAi to treat AKI. J Am Soc Nephrol. 2021;32:2467–2483. doi: 10.1681/ASN.2020111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y, Wang J, Zhang Y, Hu X, Li L, Chen P. Exosomes derived from mesenchymal stem cells ameliorate renal fibrosis via delivery of miR-186-5p. Hum Cell. 2022;35:83–97. doi: 10.1007/s13577-021-00617-w. [DOI] [PubMed] [Google Scholar]
- 77.Hao Y, Miao J, Liu W, Cai K, Huang X, Peng L. Mesenchymal stem cell-derived exosomes carry MicroRNA-125a to protect against diabetic nephropathy by targeting histone deacetylase 1 and downregulating endothelin-1. Diabetes Metab Syndr Obes. 2021;14:1405–1418. doi: 10.2147/DMSO.S286191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang A, Wang H, Wang B, Yuan Y, Klein JD, Wang XH. Exogenous miR-26a suppresses muscle wasting and renal fibrosis in obstructive kidney disease. FASEB J. 2019;33:13590–13601. doi: 10.1096/fj.201900884R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin J, Qian F, Zheng D, He W, Gong J, He Q. Mesenchymal stem cells attenuate renal fibrosis via exosomes-mediated delivery of microRNA Let-7i-5p antagomir. Int J Nanomedicine. 2021;16:3565–3578. doi: 10.2147/IJN.S299969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duan YR, Chen BP, Chen F, et al. Exosomal microRNA-16-5p from human urine-derived stem cells ameliorates diabetic nephropathy through protection of podocyte. J Cell Mol Med. 2021;25:10798–10813. doi: 10.1111/jcmm.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang H, Wang B, Zhang A, et al. Exosome-mediated miR-29 transfer reduces muscle atrophy and kidney fibrosis in mice. Mol Ther. 2019;27:571–583. doi: 10.1016/j.ymthe.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi J, Duan J, Gong H, Pang Y, Wang L, Yan Y. Exosomes from miR-20b-3p-overexpressing stromal cells ameliorate calcium oxalate deposition in rat kidney. J Cell Mol Med. 2019;23:7268–7278. doi: 10.1111/jcmm.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang B, Yao K, Huuskes BM, et al. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24:1290–1301. doi: 10.1038/mt.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang ZY, Hou YP, Zou XY, et al. Oct-4 enhanced the therapeutic effects of mesenchymal stem cell-derived extracellular vesicles in acute kidney injury. Kidney Blood Press Res. 2020;45:95–108. doi: 10.1159/000504368. [DOI] [PubMed] [Google Scholar]
- 85.Grange C, Papadimitriou E, Dimuccio V, et al. Urinary extracellular vesicles carrying klotho improve the recovery of renal function in an acute tubular injury model. Mol Ther. 2020;28:490–502. doi: 10.1016/j.ymthe.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen L, Wang Y, Li S, et al. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics. 2020;10:9425–9442. doi: 10.7150/thno.43315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi H, Kim Y, Mirzaaghasi A, et al. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality. Sci Adv. 2020;6:eaaz6980. doi: 10.1126/sciadv.aaz6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S, Lee SA, Yoon H, et al. Exosome-based delivery of super-repressor IκBα ameliorates kidney ischemia-reperfusion injury. Kidney Int. 2021;100:570–584. doi: 10.1016/j.kint.2021.04.039. [DOI] [PubMed] [Google Scholar]
- 89.Du J, Sun Q, Wang Z, et al. Tubular epithelial cells derived-exosomes containing CD26 protects mice against renal ischemia/reperfusion injury by maintaining proliferation and dissipating inflammation. Biochem Biophys Res Commun. 2021;553:134–140. doi: 10.1016/j.bbrc.2021.03.057. [DOI] [PubMed] [Google Scholar]
- 90.Choi HY, Kim TY, Lee M, et al. Kidney mesenchymal stem cell-derived extracellular vesicles engineered to express erythropoietin improve renal anemia in mice with chronic kidney disease. Stem Cell Rev Rep. 2022;18:980–992. doi: 10.1007/s12015-021-10141-x. [DOI] [PubMed] [Google Scholar]
- 91.Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Ji C, Zhang H, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv. 2022;8:eabj8207. doi: 10.1126/sciadv.abj8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srivastava A, Amreddy N, Babu A, et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci Rep. 2016;6:38541. doi: 10.1038/srep38541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan D, Zhao Y, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leidal AM, Huang HH, Marsh T, et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Biol. 2020;22:187–199. doi: 10.1038/s41556-019-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA. 2017;8:e1413. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lv F, Liu H, Zhao G, et al. Therapeutic exosomal vaccine for enhanced cancer immunotherapy by mediating tumor microenvironment. iScience. 2021;25:103639. doi: 10.1016/j.isci.2021.103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang L, Driedonks TA, Jong WS, et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. J Extracell Vesicles. 2022;11:e12192. doi: 10.1002/jev2.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou X, Miao Y, Wang Y, et al. Tumour-derived extracellular vesicle membrane hybrid lipid nanovesicles enhance siRNA delivery by tumour-homing and intracellular freeway transportation. J Extracell Vesicles. 2022;11:e12198. doi: 10.1002/jev2.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao J, Cusanovich DA, Ramani V, et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science. 2018;361:1380–1385. doi: 10.1126/science.aau0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peterson VM, Zhang KX, Kumar N, et al. Multiplexed quantification of proteins and transcripts in single cells. Nat Biotechnol. 2017;35:936–939. doi: 10.1038/nbt.3973. [DOI] [PubMed] [Google Scholar]
- 102.Stoeckius M, Hafemeister C, Stephenson W, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017;14:865–868. doi: 10.1038/nmeth.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Montesi SB, Désogère P, Fuchs BC, Caravan P. Molecular imaging of fibrosis: recent advances and future directions. J Clin Invest. 2019;129:24–33. doi: 10.1172/JCI122132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lv LL, Cao YH, Ni HF, et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305:F1220–1227. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- 106.Herrmann IK, Wood MJ, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 107.Arab T, Mallick ER, Huang Y, et al. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J Extracell Vesicles. 2021;10:e12079. doi: 10.1002/jev2.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]