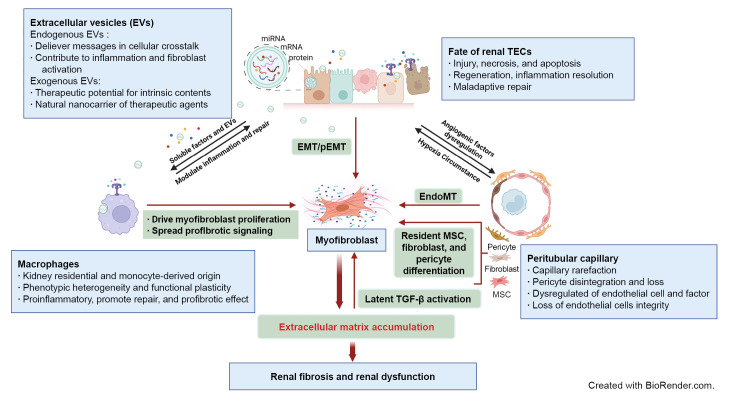
Figure 1. Schematic elucidation of cellular and signaling events in renal fibrosis.
Renal tubule injury acts as a driving force in fibrosis progression through communication with immune cells, peritubular capillary (PTC), and interstitial stroma cells via soluble or extracellular vesicle (EV) signaling. Persistent or severe injury leads to maladaptive repair of tubular epithelial cells (TECs) and subsequent EMT or pEMT, contributing to renal fibrosis. PTC rarefaction generates a hypoxic environment that promotes tubular atrophy. The phenotypic heterogeneity and functional plasticity elucidate the versatile roles of macrophages during inflammation, tissue repair, and fibrosis. Excessive accumulation of ECM components contributes to overactivation of myofibroblasts originating from multiple cellular sources and provides a substrate for latent transforming growth factor β (TGF-β) activation. Endogenous EVs play a notable role in delivery of messages in cellular communication, while exogenous EVs are being developed as new therapeutic agents for renal fibrosis.
AKI, acute kidney injury; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; EndoMT, endothelial-mesenchymal transition; MSC, mesenchymal stem cell; pEMT, partial epithelial-mesenchymal transition.