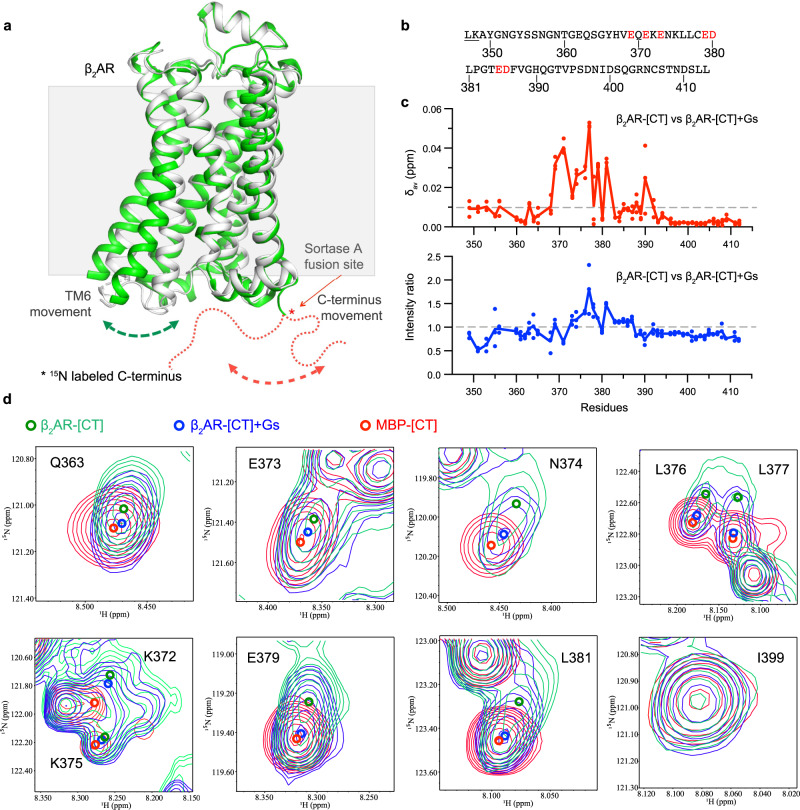
Fig. 4. The middle of the β2AR-[CT] interacts with the cytoplasmic surface of the β2AR.
a A schematic model of SrtA ligation of the 15N isotopic labeled β2AR-[CT]. The SrtA ligation site was introduced at the end of H8 (see supplementary Fig 1a). The inactive β2AR structure (PDB ID: 2RH1) is shown in the grey cartoon, while the active structure is shown in the green cartoon (PDB ID: 3P0G). b The sequence of the β2AR-[CT] labeled for NMRs studies. The red text highlights the negatively charged residues that show chemical shift changes in panel c. c The weighted average 1H–15N chemical shift changes (Δδav) and intensity ratio for each 15N β2AR-[CT] residue (347-413) in the absence and presence of Gs protein. Weighted average 1H–15N chemical shift changes were calculated as Δδav = ((ΔδH)2 + (ΔδN/5)2)1/2. The intensity ratio was calculated as IR-Gs/IR. Dots on the curve indicate two sequential 8 h of measurements of spectra and the merged spectrum. d The spectra of agonist-β2AR-[CT]-Gs complex is more similar to the spectra of MBP-[CT] than the β2AR-[CT]. Representative peaks from superposed 1H–15N HSQC spectra of 15N MBP-[CT], BI occupied 15N β2AR-[CT], and BI occupied 15N β2AR-[CT] coupled to nucleotide-free Gs. The peak centers are shown as colored circles.