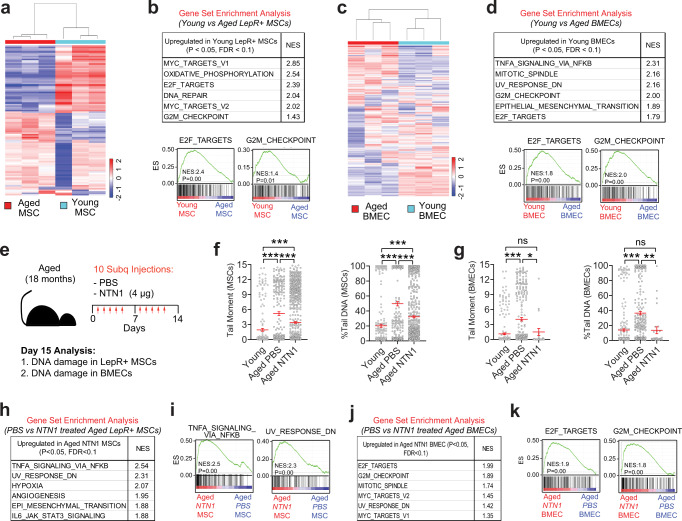
Fig. 6. NTN1 supplementation restores DNA damage within the aged BM niche.
a, b RNA-Seq analysis of LepR+ MSCs derived from young (3 month) and aged (18 month) mice. a Heatmap depicting hierarchical clustering of differentially expressed genes. b GSEA demonstrating downregulation of DDR pathways in LepR+ cells of aged mice. c, d RNA-Seq analysis of BMECs derived from young (3 month) and aged (18 month) mice. c Heatmap depicting hierarchical clustering of differentially expressed genes. d GSEA demonstrating downregulation of DDR pathways in BMECs of aged mice. e Experimental design for NTN1 treatment. f, g Alkaline comet assays demonstrating an increase in average tail-moment and % Tail DNA in both LepR+ MSCs f, and BMECs g, of aged mice as compared to young mice (N = 3 mice/group). Note that NTN1 treatment results in a significant reduction of DNA damage within aged MSCs and BMECs. Data is presented as the mean ± standard error of the mean (SEM). h–k RNA-Seq analysis of LepR+ MSCs and BMECs derived from aged (18 month) mice treated with PBS or NTN1. h, i GSEA demonstrating no significant upregulation of DDR pathways in LepR+ cells of aged mice treated with NTN1. j, k GSEA demonstrating an upregulation of DDR pathways in BMECs of aged mice treated with NTN1. Statistical significance determined using One-Way ANOVA with Tukey’s correction for multiple comparisons. *P < 0.05; **P < 0.01; ***P < 0.001. ns denotes statistically not significant.