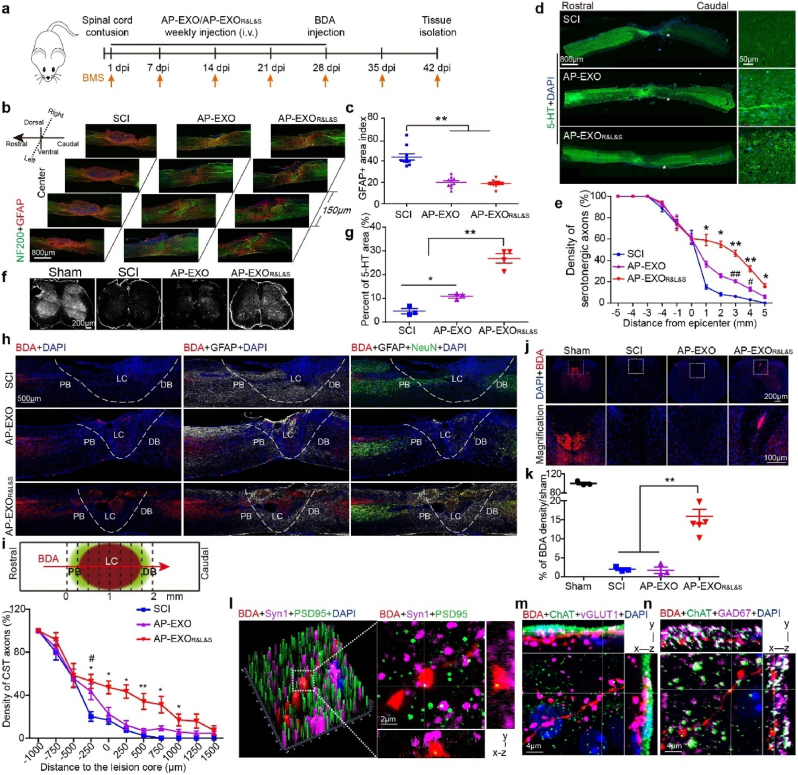
Fig. 4.
AP-EXOR&L&S enables axon regeneration after spinal cord contusion in SCI mice. Adult mice were subjected to spinal cord contusion and received intravenous injections of AP-EXO or AP-EXOR&L&S beginning at day 1 after contusion at a dose of 1 mg/kg/week for 5 weeks. Four weeks after injury, BDA was intra-cortically injected to trace CST axons and tissues were harvested 2 weeks post BDA injection. (a) Timeline of the dose regimen for AP-EXO and AP-EXOR&L&S in treating spinal cord contused mice. (b) Immunostaining of NF200-positive neuro-filaments and GFAP-positive astrocytes in serial sagittal sections of the injured area. Scale bar: 800 μm. (c) Quantification of the GFAP-positive area in the injured center (SCI: n = 10, AP-EXO: n = 10, AP-EXOR&L&S: n = 10, **p < 0.001, One way ANOVA post Student-Newman-Keuls test). (d–e) Regenerating serotonergic axons in sagittal spinal cord sections after AP-EXOR&L&S treatment. d, representative images of regenerating serotonergic axons beyond the lesion site (asterisk). Scale bar: 800 μm, inset: 50 μm. e, quantification of regenerating serotonergic axons at indicated distance beyond the lesion (SCI: n = 7, AP-EXO: n = 7, AP-EXOR&L&S: n = 7. *p < 0.05, #p < 0.05, one-way ANOVA on ranks post Tukey Test, **p < 0.001, ##p < 0.001, one-way ANOVA post Student-Newman-Keuls test, * denominated as comparison between the SCI group and the AP-EXOR&L&S group, # denominated as comparison between the SCI group and AP-EXO group). (f–g) Projection of 5-HT-positive serotonergic axons of injured mice after AP-EXOR&L&S treatment at the L1 level. f, representative images of projected serotonergic axons. Scale bar: 200 μm. g, percent area coverage of 5-HT to total L1 transection (SCI: n = 3, AP-EXO: n = 3, AP-EXOR&L&S: n = 4. *p < 0.05, **p < 0.001, one-way ANOVA post Student-Newman-Keuls test). (h) Sagittal spinal cord sections of the three treated groups showing BDA-labeled CST axons on composite tiled scans. Sections were also stained for cell nuclei (DAPI, left), astrocytes (GFAP, middle) and mature neurons (NeuN, right). Dot line demarcates astrocyte proximal (PB) and distal (DB) borders around the lesion core (LC). Scale bar: 500 μm. (i) Top, schematic of the lesion site and vertical lines used to count the density of axons crossing over each location indicated. Bottom, axon density of CST fibers. (SCI: n = 6, AP-EXO: n = 6, AP-EXOR&L&S: n = 6. *p < 0.05, #p < 0.05, **p < 0.001, −250 μm, 0 μm, 500 μm, one-way ANOVA post Student-Newman-Keuls test. 250 μm, 750 μm, 1000 μm, one-way ANOVA on ranks post Tukey test, * denominated as comparison between the SCI group and the AP-EXOR&L&S group, # denominated as comparison between the SCI group and the AP-EXO group). (j–k) Transverse spinal cord sections of projected BDA-labeled CST axons below the injury level after treatment. j, representative images of BDA-labeled CST axons below the injury site. Scale bar: 200 μm, inset: 100 μm. k, the comparison of BDA-positive CST axons below the injury site across 3 groups (sham: n = 3, SCI: n = 3, AP-EXO: n = 3, AP-EXOR&L&S: n = 6. **p < 0.001, one-way ANOVA post Student-Newman-Keuls test). (l) BDA-labeled terminals (red) beyond the lesion overlapped with the presynaptic (purple) and postsynaptic (green) markers synaptophysin (Syn1) and postsynaptic density protein 95 (PSD95), respectively. Scale bar: 2 μm. (m–n) BDA-labeled terminals (red) juxtaposed with ChAT-positive motor neurons (green) and vGLUT1-positive boutons (purple) or GAD67-positive boutons (purple) below the injury. Scale bar: 4 μm. vGLUT1, vesicular glutamate transporters. GAD67, glutamic acid decarboxylase 67.