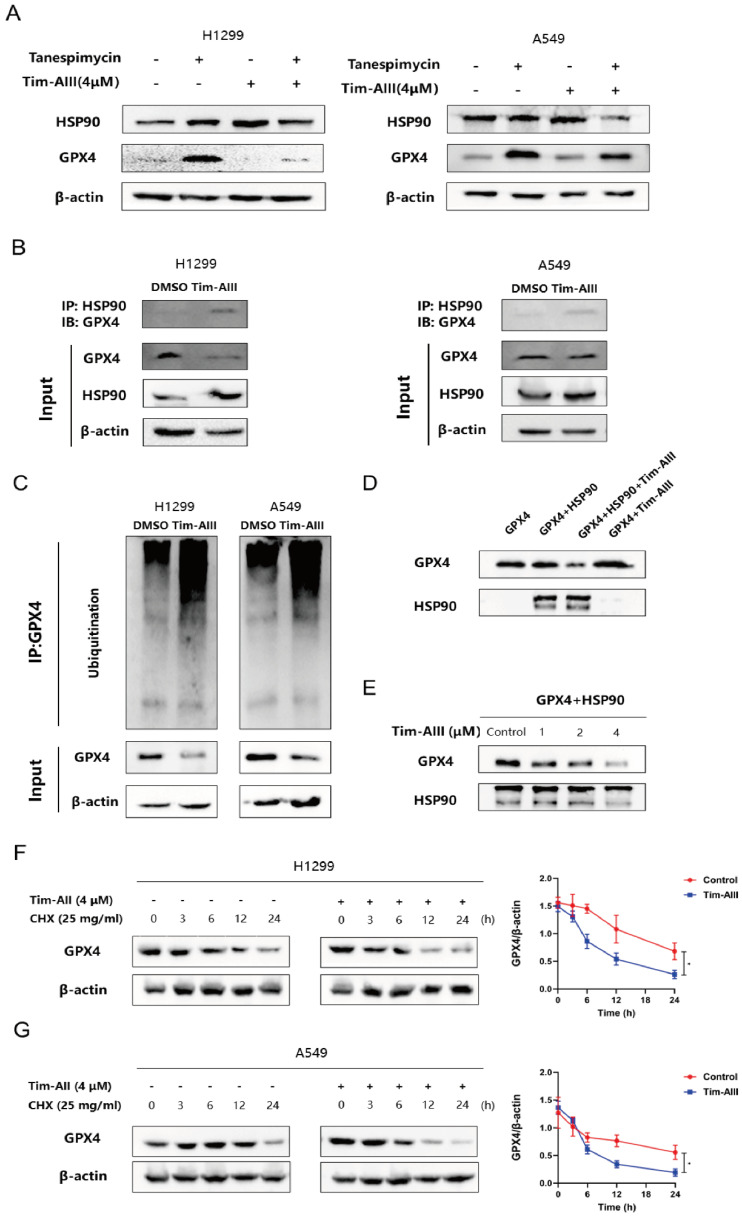
Figure 5.
Tim-AIII-HSP90 complex-induced ferroptosis in NSCLC cells was achieved by targeting and degrading GPX4. A The protein expression of HSP90 and GPX4 was examined by WB after treatment with Tim-AIII with or without tanespimycin for 48 h in H1299 and A549 cells. B Immunoprecipitation was performed to detect the interaction between GPX4 and HSP90, and WB was performed to detect HSP90 levels in the inputs and immunoprecipitates in treatment with Tim-AIII (4 μM) for 48 h in H1299 and A549 cells. C Tim-AIII was added into H1299 and A549 cells and incubated for 48 h. Then, 10 μM MG-132 was added to inhibit the protein degradation for 4 h. The proteins were collected and incubated GPX4 antibody level of shaking 4 ℃ overnight, then agarose G was added and co-incubated at 4 °C for 3h. The ubiquitination level of GPX4 was analyzed by WB assay with anti-Ubiquitination antibody. D The expression of GPX4 was examined by WB after incubating with HSP90 in the presence or absence of Tim-AIII (4 μM). E The protein expression of the GPX4 were examined by WB after incubation with HSP90 on the usage of different amounts of Tim-AIII. F WB analysis of the GPX4 expression treated with DMSO or Tim-AIII (4 μM) for the indicated time points in the presence of CHX (25 mg/mL) in H1299 cells on the left side. Quantification of GPX4 intensity, the abundance was normalized to β-actin on the right side. G WB analysis of the GPX4 expression treated with DMSO or Tim-AIII (4 μM) for the indicated time points in the presence of CHX (25 mg/mL) in A549 cells on the left side. Quantification of GPX4 intensity, the abundance was normalized to β-actin on the right side. Quantitative data were presented as mean ± SD. *p< 0.05, **p < 0.01, ***p < 0.001.