Figure 3.
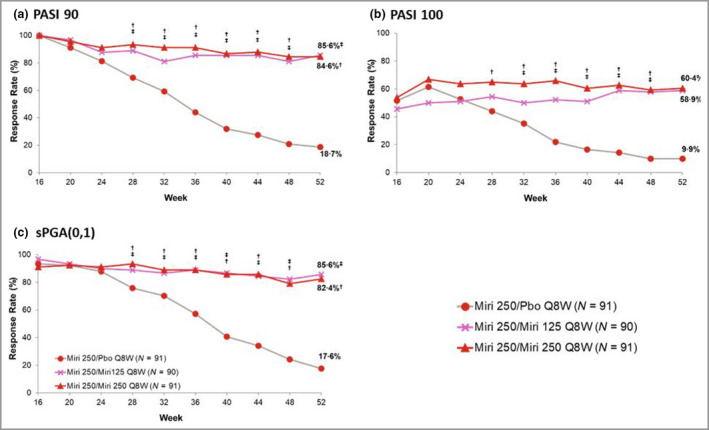
Proportion of patients maintaining or achieving (a) ≥ 90% improvement in Psoriasis Area and Severity Index (PASI 90), (b) PASI 100 or (c) static Physician’s Global Assessment (sPGA) score of 0 or 1 during the maintenance period. The time of last dose prior to randomization is week 12. Miri, mirikizumab; Pbo, placebo; Q8W, every 8 weeks. † P < 0·05 for mirikizumab 250 mg/mirikizumab 250 mg vs. mirikizumab 250 mg/placebo; ‡ P < 0·05 for mirikizumab 250 mg/mirikizumab 125 mg vs. mirikizumab 250 mg/placebo using the Cochran–Mantel–Haenszel test stratified by weight.
