Fig. 1.
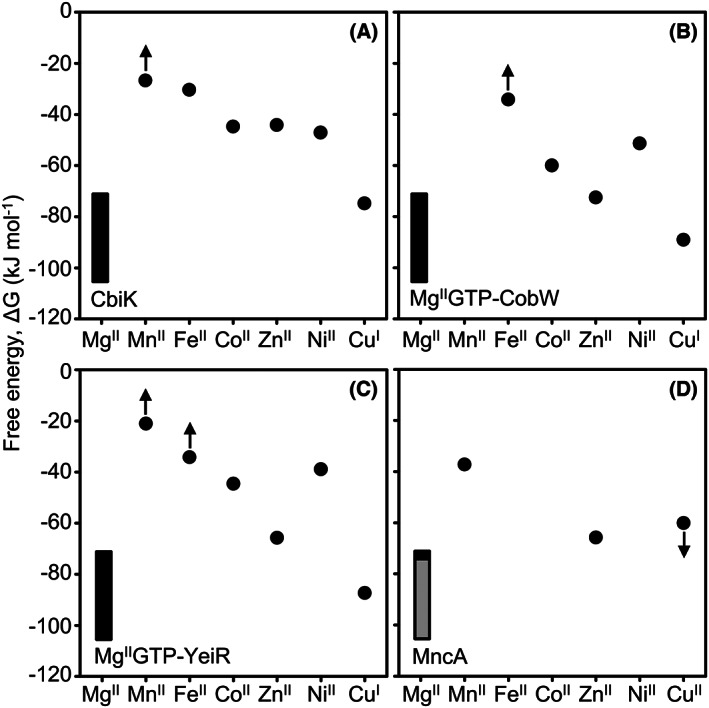
Metal binding to four proteins exemplifies the risk of mis‐metalation. Tightness of binding to the four proteins is shown for the available and exchangeable forms of metals in the cytosol (or CuII for periplasmic MncA). Values (black circles) are free energies for forming complexes calculated via the standard relationship ∆G = −RTlnK A (∆G free energy change, R molar gas constant, T temperature in kelvin, K A association constant). Note that values are logarithmically related to binding constants. The more negative the value the tighter the binding. Arrows indicate values that were at the minimum or maximum limits of the respective determinations of metal affinity. CbiK is a CoII chelatase for vitamin B12 biosynthesis [3], MgIIGTP‐CobW is a CoII metallochaperone from an alternative vitamin B12 biosynthetic pathway, MgIIGTP‐YeiR is analogous to MgIIGTP‐CobW but implicated in handling ZnII [5], MncA is a MnII cupin [4]. Values for MncA are assigned based upon competition between metals (details noted in the text). Insets show percentage occupancies with copper (black) and ZnII (grey) as a proportion of total metal occupancy. The correct metals are not the tightest binding metals and the orders of binding tend to follow the Irving–Williams series [6, 7].
