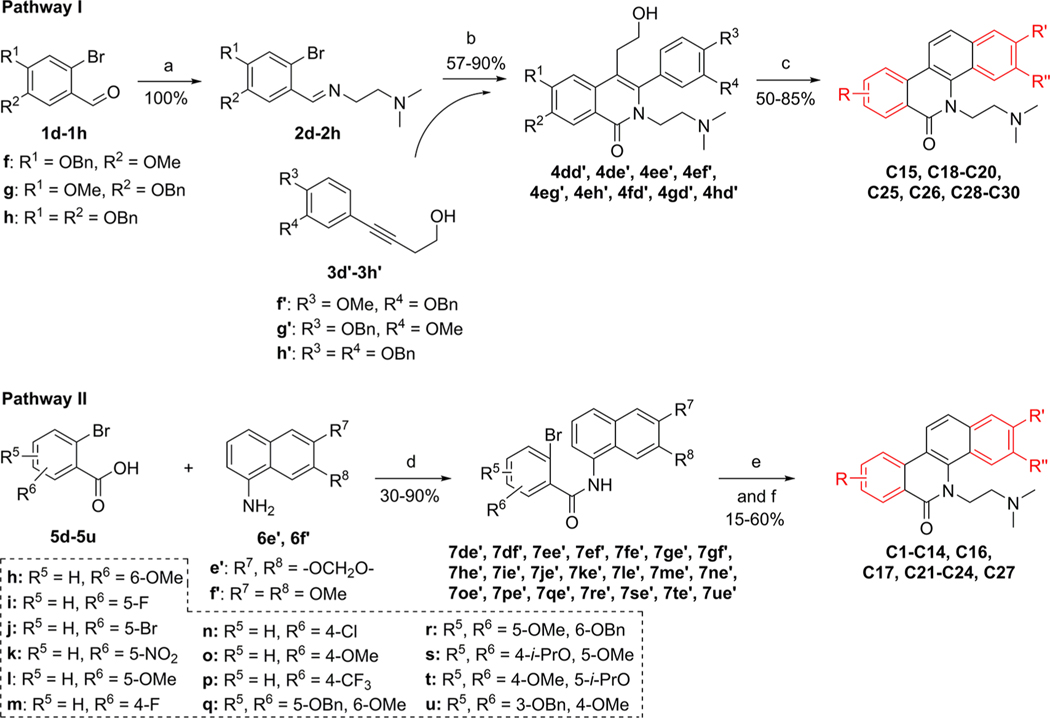
Scheme 3.
Synthesis of C1–C30a
aReagents and conditions: (a) NH2 (CH2) NMe2, MeOH, rt. (b) (i) N2, Ni(cod)2, P(o-Tol)3, MeCN, 80 °C; (ii) CsOH, K3[Fe(CN)6], MeOH, H2O, 80 °C. (c) (i) (COCl)2, DMSO, TEA, DCM, −60 °C; (ii) conc. hydrochloric acid, AcOH, 65 °C. (d) (i) SOCl2, reflux; (ii) TEA, DCM, rt. (e) (i) Cl(CH2)2NMe2/HCl, NaH, DMF, 85 °C; (ii) N2, Pd(OAc)2, P(o-tol)3, Ag2CO3, DMF, 150 °C. (f) For C12, C13 and C22, concentration hydrochloric acid, AcOH, 65 °C.