FIGURE 5.
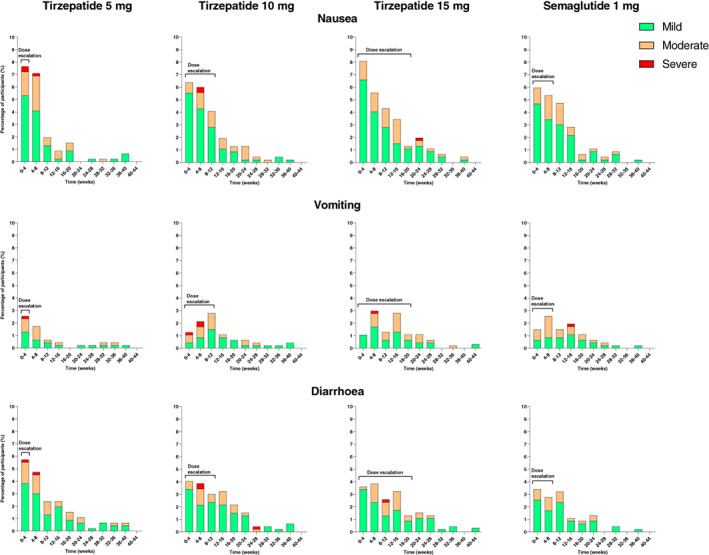
Incidence of nausea, vomiting and diarrhoea in SURPASS‐2. 56 Data are percentage of participants who reported a new event relative to participants at risk during a time interval (incidence) and from the modified intention‐to‐treat population (safety analysis set). Dose escalation indicates the time period before reaching the maintenance dose of tirzepatide 5 mg (4 weeks), 10 mg (12 weeks), and 15 mg (20 weeks) and semaglutide 1 mg (8 weeks)
