Abstract
Low socioeconomic position (SEP) may be associated with adverse outcomes in patients with myelodysplastic syndromes (MDS) inherent to for example, delayed diagnosis or reduced treatment intensity, but firm evidence is limited. In this study, we examined the association between SEP and clinical outcomes. We conducted a population‐based cohort study (2010–2018) of 2233 Danish patients with MDS. SEP measures included individual‐level information on education, cohabitation status and income retrieved from Statistics Denmark. Associations between SEP measures and disease severity at diagnosis were examined using binomial regression analysis. Using time‐to‐event analysis, we examined the association between SEP measures and treatment with allogeneic stem cell transplantation (allo‐HSCT), risk of progression to acute myeloid leukemia (AML), and death. Estimates were adjusted for covariates selected based on direct acyclic graphs and reported with 95% confidence intervals. Patients with a short education were more likely to be transfusion‐dependent at diagnosis (RR = 1.25, 95% CI: 1.04–1.45) and more likely to be diagnosed with higher risk MDS according to the International Prognostic Scoring System (RR = 1.29, 95% CI: 1.03–1.62), than patients with a long education. We found no clear association between SEP and risk of progression to AML. In adjusted models, the 1‐year risk of dying was higher in patients with short versus long education (RR = 1.34, 95% CI: 1.08–1.65), in patients with low versus high income (RR = 1.42, 95% CI: 1.14–1.77), and among patients who lived alone compared to those who lived with a partner (RR = 1.15, 0.98–1.35). These associations persisted after 3 years and 5 years of follow‐up. Notably, patients with a short education had a markedly lower rate of undergoing treatment with allo‐HSCT compared to patients with a long education (HR = 0.51, 95% CI: 0.31–0.84). In conclusion, low SEP and especially short education, were poor prognostic factors for adverse clinical outcomes among patients with MDS.
Keywords: myelodysplastic syndromes, outcomes, prognosis, socioeconomic position
1. INTRODUCTION
Myelodysplastic syndromes (MDS) encompass a group of malignant hematopoietic stem cell disorders, characterized by bone marrow failure and increased risk of anemia, life‐threatening infections, and bleeding complications. 1 Adverse prognostic factors include higher age, male sex, adverse cytogenetics, higher bone marrow blast count, bi‐ or trilinear cytopenia, transfusion dependency, therapy‐related MDS, and a higher burden of comorbidity. 1 , 2 It is recognized that low socioeconomic position (SEP) is associated with adverse outcomes among patients with cancer, 3 , 4 , 5 , 6 , 7 , 8 but most literature to date has focused on patients with solid tumors, 3 , 4 , 7 lymphoma, 5 , 9 or acute myeloid leukemia (AML). 6 , 10 Few studies examined the prognostic impact of SEP among patients with MDS. 11 , 12 , 13 , 14 Importantly, existing studies yielded conflicting results, 11 , 12 , 13 , 14 and were limited by insufficient sample size, 13 , 14 inclusion of patients >66 years only, 12 and were based on single‐center data. 13 , 14 Further, studies lacked detailed and valid measures of SEP. 12 , 13 , 14 In addition, disease severity at diagnosis and important clinical outcomes, for example, progression to AML and treatment with allogeneic hematopoietic stem cell transplantation (allo‐HSCT) were less studied.
More firm evidence on the prognostic impact of SEP in MDS patients is necessary to inform clinicians, patients, and policy makers and to guide development of future intervention strategies to address inequalities in MDS treatment and care. We therefore investigated the association between individual‐level markers of SEP (education, income, and cohabitation status) and disease severity at diagnosis, curative‐intent treatment with allo‐HSCT, risk of progression to AML, and mortality in an unselected population of MDS‐patients.
2. METHODS
2.1. Setting
The Danish Health care system is tax‐funded guaranteeing free access to primary‐and hospital care for all Danish citizens (≈6.0 million). 15 At birth or upon immigration all Danish citizens are assigned a unique 10‐digit identification number by the Danish Civil Registration System. 16 This identifier allows longitudinal follow‐up and unambiguous linkage between registries.
2.2. Design and study population
We conducted a nationwide population‐based cohort‐study. We identified all patients with incident MDS in the Danish Myelodysplastic Syndromes Database (DMDSD) between 1 January 2010 and 31 December 2018. The DMDSD is a nationwide clinical cancer database including more than 98% of Danish patients with incident MDS since 2010. 17 , 18 It holds detailed individual‐level clinical and diagnostic information and a recent validation study showed high validity of registered data in the DMDSD. 18 MDS patients are treated at 10 hospitals in Denmark of which six are permitted to treat MDS with disease‐modifying agents, or remission‐induction chemotherapy, while four regional hospital departments treat MDS patients with best supportive care only. Allo‐HSCT, which is the only curative‐intent approach in MDS, is performed at two highly specialized centers. No MDS patients are treated at private hospitals and transportation to and from the hospital during treatment and follow‐up is free of charge. An overview of study design and how we included the different study variables according to the underlying time‐scale is graphically illustrated in Supplementary Figure S1 and described below.
2.3. Exposure/socioeconomic data
Individual‐level information on education, income, and cohabitation status were retrieved from registries at Statistics Denmark. 19 , 20 , 21 We chose education as a measure of knowledge‐related resources and categorized the highest attained level of education according to ‘the International Standard Classification of Education Codes, 22 as short education (≤9 years of mandatory primary school), medium education (10–12 years of schooling corresponding to upper secondary school and vocational education), and long education (>12 years). We chose individual‐level patient income as a measure of material resources. Income was grouped into quintiles, based on the annual median age and sex‐adjusted income of the entire population, with low income denoting the quintile with lowest income and high income the quintile with highest income. Cohabitation status was chosen as a measure of social support and was defined as living alone or living with a partner.
2.4. Outcomes
We defined disease severity at diagnosis by level of prognostic risk group according to the ‘International Prognostic Scoring System’ (IPSS), the revised IPSS (IPSS‐R) and transfusion dependency. 2 Using baseline data on level of hemoglobin, platelet count, white blood cell count, blast count and karyotype we calculated the IPSS risk score and the revised IPSS risk score. According to the IPSS risk score we categorized patients into IPSS low‐risk MDS (low‐risk and intermediate‐1) and IPSS high‐risk MDS (intermediate‐2 and high‐risk). For the IPSS‐R risk score we categorized patients into IPSS‐R low‐risk MDS (very low and low), IPSS‐R intermediate‐risk MDS and IPSS‐R high‐risk MDS (high and very high). 2 If patients had missing data on any of the baseline data used to calculate the IPSS or IPSS‐R risk score no risk score or prognostic group was calculated (missing data). Complete transfusion information including date and type of product was obtained from the Danish Transfusion Database. 23 In Denmark, packed red blood cells are stored in SAGM (saline‐adenine‐glucose‐mannitol) and one unit has a volume of approximately 250 ml. We examined transfusion status at MDS diagnosis by including data on units of packed red blood cell transfusions (0, 1, 2 or >2 transfusions) administered within 56 days prior to diagnosis. Patients were considered transfusion dependent at diagnosis if they had received one or more packed red blood cell transfusions within this period. Information on treatment with allo‐HSCT was obtained from the Danish National Patient Registry (DNRP) using the procedure codes specified in Table S1. The DNRP holds information on in‐hospital treatment‐related procedure codes since 1999 24 and procedure codes on allo‐HSCT in the DNRP were recently validated showing a positive predictive value of 99%. 25 Information about date of progression to AML was obtained from the Danish National Acute Leukemia Registry, which is a clinical cancer database that includes nearly 100% of all Danish patients with AML since 1 January 2000. 26 To ascertain mortality, we used the Danish Civil Registration System which holds daily updated information on each patient's vital status and date of death since 1968. 16
2.5. Clinical information/covariates
Information on age, sex, bone marrow blast count, laboratory values (hemoglobin in g/dl, platelet count × 109/L, white blood cell count × 109/L) and type of MDS (de novo/therapy‐related), were retrieved from the DMDSD. International Classification of Diseases 10th edition (ICD‐10) codes on comorbidities were obtained from the DNRP within a 10 year period prior to the date of diagnosis with MDS. The DNRP holds valid data on discharge diagnoses from all Danish hospitals since 1977 and covers both inpatient and outpatient hospital contacts. 24 We obtained data on main comorbidities and we computed the Charlson Comorbidity Index (CCI) score for each patient, excluding myeloid diseases from the index. The CCI score was categorized into 3 levels: score of 0; score of 1–2, and score ≥3. 27 ICD‐10 codes on main comorbidities and comorbidities included in the CCI are provided in Supplementary Table S1.
2.6. Statistical analyses
All MDS‐patients started contributing person‐time on the day of MDS diagnosis and were followed until emigration, death, or 31 December 2019, whichever came first. To compute cumulative risks and to graphically illustrate risks of study outcomes by exposure status, we used the Kaplan‐Meier method and cumulative incidence risk function (for non‐fatal outcomes), accounting for death as competing risk. 28
To decide which variables to include in our multivariable models, we created three directed acyclic graphs (DAG) of assumed causal associations between level of education, income, and cohabitation status and risk of progression to AML and death, treatment with allo‐HSCT and disease severity at diagnosis (Supplementary Figure S2–S4). Based on the DAGs, we conditioned each analysis on the following: education and cohabitation status on age and sex; income on age, sex, CCI, education, employment, and cohabitation status. We considered MDS characteristics and treatment modalities as potential mediators on the causal pathway, and these were therefore not adjusted for in line with recommendations by Lash et al. 28
2.7. Disease severity at diagnosis
Using binomial regression analysis, we assessed the risk of being transfusion‐dependent at diagnosis or being diagnosed with higher risk MDS by exposure status. Results were presented as absolute risks and adjusted relative risks.
2.8. Transfusion burden within the first year after diagnosis
To describe the transfusion burden within the first year after MDS diagnosis by exposure group we defined three categories of transfusion burden according to the International Working Group response criteria from 2018 29 : (1) no transfusion burden (NTB), including patients who received ≤2 packed red blood cell units in a period of 16 weeks (2) low transfusion burden (LTB) defined as 3–7 packed red blood cell units in a period of 16 weeks and high transfusion burden (HTB) defined as ≥8 packed red blood cell units in a 16 weeks' period. In addition, we calculated incidence rates (IR) of transfusions within the first year after diagnosis per person year by dividing the number of units of packed red blood cells by risk time stratified by exposure group. We also used Poisson regression models to compute crude and adjusted 1‐year‐incidence rate ratios of transfusions by exposure group.
2.9. Curative intent treatment with allo‐HSCT
Using Cox proportional hazard regression analysis, we calculated crude and adjusted hazard ratios (HRs) for the association between level of education, income and cohabitation status and treatment with allo‐HSCT. The Cox proportional hazards model assumptions were graphically verified using log minus log plot.
2.10. Relative risk of progression to AML and death
Crude and adjusted relative risks of progression to AML and death were computed using the pseudovalues approach 30 and estimates were reported with 95% confidence intervals. Analyses were also stratified by age group (±75 years), sex, calendar year (2010–2013 and 2014–2018), IPSS risk score (low‐risk and high‐risk), IPSS‐R risk score (low‐risk, intermediate‐risk and high‐risk), transfusion status (transfusion‐naive and ≥1 transfusions), and CCI (0, 1–2, >2).
The study was approved by the Danish Data Protection Agency (record number: 1‐16‐02‐321‐18) and according to Danish law, no approval from an ethical committee was required. All analyses were conducted using Stata statistical software version 14 (StataCorp LP, College Station, Texas; www.stata.com).
3. RESULTS
Our study‐cohort comprised 2330 MDS patients. We excluded 97 patients who had either missing information on exposures, a MDS diagnosis made upon autopsy, or a history of immigration within five years prior to the MDS diagnosis. The latter to ensure that patients had incident MDS and to ensure a complete medical history of comorbidities. Thus, our final study‐cohort included 2233 MDS patients. Overall, median age at diagnosis was 75 years (interquartile range [IQR] 68–81) and 1399 (63%) were male. A total of 480 (22%) patients had high‐risk MDS according to IPSS and 360 (16%) patients had high‐risk MDS according to IPSS‐R. Patient and disease characteristics by exposure status are provided in Table 1.
TABLE 1.
Baseline patient demographics and disease characteristics of 2233 patients diagnosed with MDS in Denmark during 2010–2018
| Education a | Cohabitation status | Income b | ||||||
|---|---|---|---|---|---|---|---|---|
| Short | Medium | Long | Living alone | Living with a partner | Low | Medium | High | |
| Number | 716 | 1098 | 419 | 875 | 1358 | 457 | 1312 | 464 |
| Age, median (25th–75th percentiles) | 78 (72–84) | 73 (67–80) | 74 (67–80) | 78 (70–83) | 74 (68–80) | 75 (68–81) | 75 (68–81) | 76 (69–82) |
| Male, no. (%) | 409 (57) | 712 (65) | 278 (66) | 410 (47) | 989 (73) | 286 (63) | 803 (61) | 310 (67) |
| Bone marrow blast count, median (IQR) | 3 (0–7) | 2 (1–6) | 2 (0–6.5) | 2 (0–6) | 3 (0–7) | 3 (1–8) | 2 (0–6) | 2 (0–6) |
| Hemoglobin, g/dL, median (IQR) | 9.8 (8.7–11.0) | 9.8 (8.7–11.1) | 10.0 (8.9–11.4) | 9.8 (8.7–11.0) | 10.0 (8.9–11.3) | 9.7 (8.7–10.8) | 10.0 (8.9–11.3) | 9.9 (8.7–11.3) |
| Platelet count, x 109/L, median (IQR) | 116 (69–228) | 132 (70–245) | 116 (65–212) | 132 (68–257) | 122 (70–221) | 115 (62–215) | 126 (73–241) | 127 (67–232) |
| WBC, x 109/L, median (IQR) | 1.9 (0.9–3.7) | 1.9 (1.0–3.5) | 1.7 (0.9–3.0) | 2.0 (1.0–3.8) | 1.7 (0.9–3.3) | 1.9 (1.0–3.5) | 1.8 (0.9–3.5) | 1.7 (0.9–3.3) |
| Therapy related MDS, no. (%) | 65 (9) | 118 (11) | 46 (11) | 77 (9) | 152 (11) | 56 (12) | 125 (10) | 48 (10) |
| IPSS‐R, no. (%) | ||||||||
| Very low | 80 (11) | 170 (15) | 65 (16) | 116 (13) | 199 (15) | 47 (10) | 205 (16) | 63 (14) |
| Low | 163 (23) | 277 (25) | 101 (24) | 212 (24) | 329 (24) | 99 (22) | 326 (25) | 116 (25) |
| Intermediate | 97 (14) | 139 (13) | 49 (12) | 109 (12) | 176 (13) | 60 (13) | 161 (12) | 64 (14) |
| High | 55 (8) | 97 (9) | 38 (9) | 69 (8) | 121 (9) | 46 (10) | 97 (7) | 47 (10) |
| Very high | 67 (9) | 73 (7) | 30 (7) | 60 (7) | 110 (8) | 50 (11) | 88 (7) | 32 (7) |
| Missing | 254 (35) | 342 (31) | 136 (32) | 309 (35) | 423 (31) | 155 (34) | 455 (33) | 142 (30) |
| IPSS, no. (%) | ||||||||
| Low | 137 (19) | 248 (23) | 99 (22) | 197 (23) | 281 (21) | 90 (20) | 287 (22) | 101 (22) |
| Int‐1 | 278 (39) | 466 (42) | 185 (44) | 349 (40) | 580 (43) | 175 (38) | 561 (43) | 193 (42) |
| Int‐2 | 119 (17) | 150 (14) | 63 (15) | 122 (14) | 210 (15) | 79 (17) | 178 (14) | 75 (16) |
| High | 52 (7) | 73 (7) | 23 (5) | 58 (7) | 90 (7) | 45 (10) | 85 (6) | 18 (4) |
| Missing | 130 (18) | 161 (15) | 55 (13) | 149 (17) | 197 (15) | 68 (15) | 201 (15) | 77 (17) |
| Number of RBC transfusions c | ||||||||
| 0 | 453 (63) | 749 (68) | 300 (72) | 558 (64) | 944 (70) | 290 (63) | 909 (69) | 303 (65) |
| 1 | 100 (14) | 128 (12) | 41 (10) | 121 (14) | 148 (11) | 58 (13) | 150 (11) | 61 (13) |
| 2 | 79 (11) | 96 (9) | 42 (10) | 90 (10) | 127 (9) | 51 (11) | 119 (9) | 47 (10) |
| >2 | 84 (12) | 125 (11) | 36 (9) | 106 (12) | 139 (10) | 58 (13) | 134 (109 | 53 (11) |
| Comorbidity, no. (%) | ||||||||
| Ischemic heart disease | 108 (15) | 182 (17) | 50 (12) | 119 (14) | 221 (16) | 74 (16) | 175 (13) | 91 (20) |
| Congestive heart failure | 71 (10) | 99 (9) | 36 (9) | 88 (10) | 118 (9) | 44 (9) | 113 (9) | 49 (11) |
| Chronic pulmonary disease | 104 (15) | 133 (12) | 36 (9) | 122 (14) | 151 (11) | 66 (14) | 161 (12) | 46 (10) |
| Moderate to severe renal disease | 39 (6) | 61 (6) | 18 (4) | 47 (5) | 71 (5) | 29 (6) | 64 (5) | 25 (5) |
| Solid tumor | 119 (17) | 182 (17) | 76 (18) | 130 (15) | 247 (18) | 78 (17) | 210 (16) | 89 (19) |
| Alcohol related disorders | 20 (3) | 41 (4) | 12 (3) | 34 (4) | 39 (3) | 17 (4) | 39 (3) | 17 (4) |
| CCI score, no. (%) | ||||||||
| 0 | 409 (57) | 642 (58) | 266 (64) | 506 (58) | 816 (60) | 251 (55) | 795 (61) | 271 (58) |
| 1–2 | 215 (30) | 327 (30) | 102 (24) | 254 (29) | 395 (29) | 139 (30) | 376 (29) | 129 (28) |
| ≥3 | 92 (13) | 129 (12) | 51 (12) | 115 (13) | 158 (12) | 67 (14) | 141 (11) | 64 (14) |
Abbreviations: AML, acute myeloid leukemia; CCI, Charlson Comorbidity Index; IPSS, International Prognostic Scoring System; IQR, interquartile range; No, number; RBC, red blood cells.
Education: short ≤9 years, medium 10–12 years, low ≥12 years.
Each individual was assigned to categorical indicators for income ranked from low income to high income. Income was adjusted for birth year and sex.
Number of RBC transfusions administered within 56 days prior to MDS diagnosis.
3.1. Risk of being transfusion‐dependent or being diagnosed with higher‐risk MDS at time of diagnosis
Patients with short education were more likely transfusion‐dependent at diagnosis (RR = 1.25, 95% CI: 1.04–1.45), and had higher risk of being diagnosed with high‐risk MDS according to IPSS (RR = 1.29, 95% CI: 1.03–1.62), and higher risk of being diagnosed with intermediate‐risk and high‐risk MDS according to IPSS‐R (RR = 1.18, 95% CI: 1.00–1.40). Living alone increased the risk of being transfusion‐dependent at diagnosis (RR = 1.15, 95% CI: 1.01–1.30), while we found no association between income and transfusion‐dependency. Neither cohabitation status nor income was clearly associated with adverse IPSS/IPSS‐R risk score (Table 2).
TABLE 2.
Association between level of education, cohabitation status and income and risk of being transfusion‐dependent at diagnosis or being diagnosed with high‐risk MDS according to IPSS or intermediate– and high‐risk MDS according to IPSS‐R
| Transfusion‐dependent at diagnosis a | High‐risk MDS according to IPSS | Intermediate/high‐risk MDS according to IPSS‐R | ||||
|---|---|---|---|---|---|---|
| Prevalence, (%) | Adjusted RR, (95% CI) b | Prevalence, (%) | Adjusted RR, (95% CI) b | Prevalence, (%) | Adjusted RR, (95% CI) b | |
| Education | ||||||
| Long | 119/419 (28%) | Ref. | 86/419 (21) | Ref. | 117/283 (41%) | Ref. |
| Medium | 349/1098 (32%) | 1.12 (0.94–1.33) | 223/1098 (20) | 1.01 (0.81–1.26) | 309/756 (41%) | 1.00 (0.85–1.17) |
| Short | 263/716 (38%) | 1.25 (1.04–1.45) | 171/716 (24) | 1.29 (1.03–1.62) | 219/462 (47%) | 1.18 (1.00–1.40) |
| Cohabitation status | ||||||
| Living with a partner | 414/1358 (30%) | Ref. | 300/1358 (22) | Ref. | 407/935 (44%) | Ref. |
| Living alone | 317/875 (36%) | 1.15 (1.01–1.30) | 180/875 (21) | 0.98 (0.83–1.16) | 238/566 (42%) | 0.98 (0.87–1.12) |
| Income | ||||||
| High | 167/457 (37%) | Ref. | 93/464 (20) | Ref. | 143/322 (44%) | Ref. |
| Medium | 403/1312 (31%) | 0.84 (0.73–0.98) | 263/1312 (20) | 0.86 (0.74–1.00) | 346/877 (39%) | 0.91 (0.74–1.13) |
| Low | 161/464 (35%) | 1.01 (0.84–1.20) | 124/457 (27) | 1.12 (0.95–1.33) | 156/302 (52%) | 1.16 (0.91–1.47) |
Note: 346 (15%) patients had missing data on IPSS prognostic score and 732 (33%) patients had missing data on IPSS‐R prognostic score.
Abbreviations: CI, confidence interval; IPSS, International prognostic Scoring System; IPSS‐R, International Prognostic Scoring System‐Revised; RR, relative risk.
Transfusion dependency at diagnosis was defined as having received one or more packed red blood cells within 56 days prior to MDS diagnosis.
Education and cohabitation status were adjusted for age and sex while income was adjusted for age, sex, CCI score, education, employment and cohabitation status.
3.2. Transfusion burden within the first year after diagnosis
Median number of transfusions within the first year after diagnosis for patients with short education were 4 (0–13) compared to 2 (0–14) for patients with a long education. Patients with a short education were less likely to have NTB compared to patients with a long education (47% vs. 55%), they had a higher incidence rate of transfusions (10.8 per person year vs. 9.5 per person year), and a higher incidence rate ratio of transfusions within the first year after diagnosis (IRR = 1.21, 95% CI: 1.16–1.26). The same pattern was seen for patients with low income while we found no association between cohabitation status and transfusion use (Supplementary Table S2).
3.3. Probability of curative intent treatment with allo‐HSCT
During follow‐up, 161 (7%) patients underwent allo‐HSCT. In adjusted models, the HR of undergoing allo‐HSCT was markedly lower among patients with a short versus long education (HR = 0.51, 95% CI: 0.31–0.84) (Figure 1 and Supplementary Table S3). There were no clear association between cohabitation status or income and probability of undergoing allo‐HSCT.
FIGURE 1.
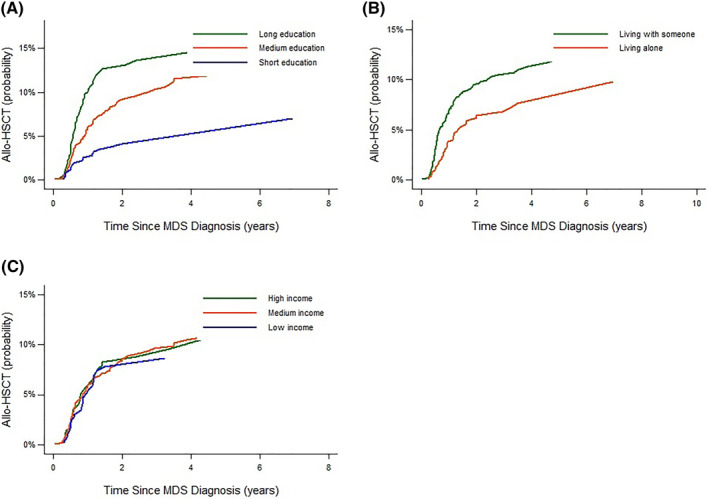
Cumulative probability of treatment with allogeneic stem cell transplantation (allo‐HSCT) stratified by level of education (A), cohabitation status (B) and by level of income (C)
3.4. Risk of progression to AML
Within the follow‐up period, 240 MDS patients progressed to AML. The 1‐year cumulative risk of progression to AML were similar across education levels and income groups. For patients who lived alone the 1‐year cumulative risk of progression to AML was slightly lower compared to patients who lived with a partner (4.6% vs. 6.7%) and this pattern persisted both three and 5 years after diagnosis (Figure 2 and Supplementary Table S4). However, in adjusted models, there were no clear association between exposure status and risk of progression to AML (Table 3).
FIGURE 2.
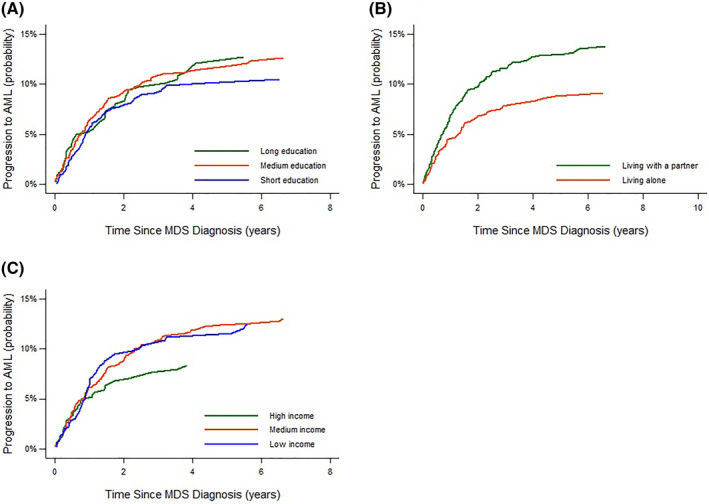
Cumulative risk of progression to acute myeloid leukemia (AML) stratified by level of education (A), cohabitation status (B) and by level of income (C)
TABLE 3.
1‐year, 3‐year and 5‐year risks of progression to acute myeloid leukemia (AML) and death following a first‐time MDS‐diagnosis. Unadjusted and adjusted relative risks. Data are shown by level of education, cohabitation status and income
| 1‐year relative risk, (95% CI) | 3‐year relative risk, (95% CI) | 5‐year relative risk, (95% CI) | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted a | Unadjusted | Adjusted a | Unadjusted | Adjusted a | |
| Progression to AML | ||||||
| Education | ||||||
| Long | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Medium | 1.21 (0.76–1.93) | 1.19 (0.72–1.98) | 1.10 (0.78–1.56) | 1.16 (0.81–1.68) | 0.97 (0.70–1.34) | 0.99 (0.70–1.54) |
| Short | 1.04 (0.62–1.73) | 1.23 (0.69–2.18) | 0.93 (0.64–1.37) | 1.17 (0.77–1.78) | 0.84 (0.59–1.20) | 1.04 (0.71–1.54) |
| Cohabitation status | ||||||
| Living with a partner | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Living alone | 0.68 (0.47–0.98) | 0.83 (0.51–1.33) | 0.68 (0.51–0.90) | 0.79 (0.58–1.07) | 0.68 (0.52–0.89) | 0.78 (0.59–1.05) |
| Income | ||||||
| High | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Medium | 1.23 (0.78–1.93) | 1.07 (0.58–1.98) | 1.41 (0.98–2.03) | 1.35 (0.88–2.09) | 1.47 (1.04–2.09) | 1.43 (0.95–2.16) |
| Low | 1.24 (0.72–2.11) | 1.17 (0.54–2.53) | 1.38 (0.90–2.11) | 1.46 (0.85–2.52) | 1.32 (0.87–2.00) | 1.45 (0.89–2.37) |
| Death | ||||||
| Education | ||||||
| Long | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Medium | 1.00 (0.81–1.25) | 1.00 (0.80–1.23) | 1.13 (1.00–1.28) | 1.11 (0.99–1.25) | 1.11 (1.00–1.24) | 1.11 (1.01–1.23) |
| Short | 1.52 (1.23–1.88) | 1.34 (1.08–1.65) | 1.33 (1.17–1.51) | 1.18 (1.05–1.34) | 1.32 (1.19–1.47) | 1.22 (1.11–1.35) |
| Cohabitation status | ||||||
| Living with a partner | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Living alone | 1.26 (1.08–1.45) | 1.15 (0.98–1.35) | 1.12 (1.03–1.22) | 1.06 (0.98–1.15) | 1.08 (1.01–1.16) | 1.03 (0.97–1.10) |
| Income | ||||||
| High | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Medium | 0.77 (0.64–0.92) | 0.74 (0.60–0.90) | 0.94 (0.84–1.04) | 0.94 (0.85–1.04) | 0.97 (0.89–1.06) | 0.95 (0.88–1.02) |
| Low | 1.41 (1.16–1.71) | 1.37 (1.10–1.71) | 1.15 (1.02–1.29) | 1.12 (1.00–1.26) | 1.12 (1.01–1.23) | 1.06 (0.97–1.16) |
Abbreviation: CI, confidence interval.
Education and cohabitation status were adjusted for age and sex and income was adjusted for age, sex, CCI score, education, employment and cohabitation status.
3.5. Risk of all‐cause mortality
During follow‐up (median 1.9 years, IQR: 1.0–3.8) 1426 patients died (64%). The 1‐year, 3‐year, and 5‐year cumulative risk of death were increased for patients with a short education compared to patients with a long education, for patients with low income compared to patients with a high income and for patients living alone compared to patients living with a partner (Figure 3 and Supplementary Table S4). In adjusted models, the risk of death one year from diagnosis was higher in patients with short vs. long education (RR = 1.34, 95% 1.08–1.65), in patients with low versus high income (RR = 1.37, 95% CI: 1.10–1.71), and among patients who were living alone compared to those who lived with a partner (RR = 1.15, 95% CI: 0.98–1.35). The increased risk of death among patients with short education persisted after both 3 years and 5 years of follow‐up while it tapered off for patients with low income and for patients living alone (Table 3).
FIGURE 3.
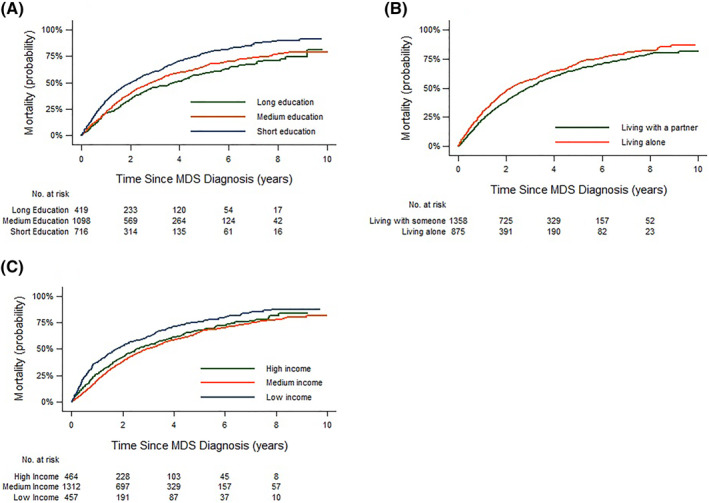
All‐cause mortality stratified by level of education (A), cohabitation status (B) and by level of income (C)
3.6. Stratified analyses
The stratified analyses are shown in Supplementary Table S5–S11. Due to imprecise effect estimates, firm conclusions on subgroup analyses on the risk of progression to AML could not be drawn (data not shown). The association between level of education, cohabitation status, and risk of death was stronger among MDS patients below 75 years (Table S5), whereas the association between level of income and risk of death was only evident in older MDS patients and only one year and three years after MDS diagnosis. The association between education level and 1‐year mortality was furthermore broadly similar among men and women, but while the association tapered off for women, it persisted during five years for men (Table S6). Within strata of transfusion‐status, IPSS and IPSS‐R risk score results were broadly similar to our main‐findings (Supplementary Table S8–S10). Results within strata of IPSS‐R, should however, be interpreted with caution due to imprecise effect estimates. The association between education, cohabitation status, and income and risk of death was furthermore evident among patients with a CCI ≤2 while it diminished in patients with a CCI >2 (Supplementary Table S11).
4. DISCUSSION
In this population‐based study of more than 2000 MDS patients, short education, living alone, and low income were associated with increased all‐cause mortality. These associations were strongest within 1 year after diagnosis, were more pronounced in younger than older MDS patients, and they were more evident among men than women. We found no clear association between exposure status and risk of progression to AML. Patients with short education were more likely to be transfusion‐dependent at diagnosis, had a higher transfusion need within the first year after diagnosis, and had more advanced disease at diagnosis based on IPSS and IPSS‐R than those with long education. Notably, the rate of allo‐HSCT was markedly lower among short‐educated patients compared to patients with a long education.
Our study significantly expands the literature on the prognostic impact of SEP among patients with MDS. However, because the definition of SEP varies considerably and is highly dependent on the available data and the underlying source population, direct comparison of our findings to prior studies is challenging. 31 , 32 , 33 , 34
Our findings on the association between SEP and mortality are in line with two prior studies. 11 , 12 A Swedish study n = 2945, using individual‐level information on education, income and cohabitation status, found that the risk of death was 50% higher in the lowest income quintile compared to the highest (HR = 1.5, 95% CI: 1.3–1.8) and 40% higher among patients with a short education compared to patients with a long education (HR = 1.4, 95% CI: 1.2–1.6). 11 An older ‘Surveillance Epidemiology and End Results’ (SEER) based US study n = 2118, using neighborhood socioeconomic status as a measure of SEP, reported that patients from low SEP neighbourhoods had increased risk of death compared to patients residing in high‐SES neighbourhoods (HR = 1.17, 95% CI: 1.02–1.34). Two small single‐center studies, 13 , 14 however, failed to demonstrate any association between SEP and mortality in MDS patients. This may be explained by different SEP measures and the lack of relative adjusted effect estimates.
Several mechanisms may underlie our findings. One potential explanation of the higher all‐cause mortality among patients with low SEP may be a delayed diagnosis and thereby more advanced MDS at time of diagnosis. Data on the association between SEP and disease characteristics at time of diagnosis are scarce. For instance, the Swedish study reported that a higher proportion of patients in the lower income quintiles were transfusion dependent at diagnosis compared to patients in the highest income quintile (48%–52% vs. 36%), but they did not analyze this disproportionality any further. Like‐wise, The SEER study found that patients with high SEP were more likely to be diagnosed with refractory anemia and refractory anemia with ringsideroblasts, that is, MDS subtypes known to have longer survival 1 compared to patients with lower SEP but no effect estimates were reported. Additionally, a single‐center European study (n = 283) 14 found that 25% of patients in the lowest SEP quintile based on the Scottish Index of Multiple Deprivation were diagnosed with higher‐risk MDS versus 18% in the highest SEP quintile but they reported no association between SEP quintiles and IPSS at diagnosis based on the Chi Squared (x 2) test. This analysis is, however, not suitable for such a comparison as it does not account for, for example, differences in the age and sex distribution, which may confound results. Thus, our findings and results from prior larger studies may indicate an inadequate health‐seeking behavior among patients with lower SEP leading to more advanced MDS at time of diagnosis. The higher all‐cause mortality among patients with lower SEP may therefore partly be explained by lead time bias—as patients with higher SEP may be diagnosed with MDS at an earlier stage making it look like they survive longer. Nonetheless, this is unlikely to fully explain our findings as the direction of the associations persisted in strata of transfusion dependency, IPSS and IPSS‐R.
Another mechanism that may contribute to the higher mortality among patients with low SEP may be related to the treatment of MDS. A Swedish study also examined the probability of treatment with allo‐HSCT as a function of income, education, and cohabitation status. Despite not accounting for the competing risk of death, they found, consistent with our results, a notably lower transplant rate among patients with shorter education (RR = 0.7, 95% CI: 0.5–1.0), patients living alone (RR = 0.7, 95% CI: 0.5–0.9), and in patients with lower income (RR = 0.3, 95% CI: 0.2–0.3). As such, our results and those from the Swedish study suggest that SEP may influence the decision process of allocating patients to allo‐HSCT.
In addition to all‐cause mortality, we examined the association between exposure status and risk of progression to AML, which so far only have been studied sparsely. 13 , 14 In line with our findings, a European study found no association between SEP quintiles and the risk of progression to AML. In contrast, a Canadian study found an increased risk of progression to AML among patients with higher income. 13 Results from these studies should, however, be interpreted with caution due to limited statistical analysis as they did not account for the competing risk of death.
Our findings have important implications. Of the three socioeconomic measures used in this study, short education was consistently associated with adverse outcomes. As education reflects material and intellectual resources and may mirror the ability to understand health‐related messages, communicate with health‐care professionals, and react timely to disease‐related symptoms, free access to hospitals and free treatment, may not be sufficient to nullify inequalities in cancer outcomes. In order to prevent the gap in health‐seeking behavior and delayed diagnosis, ongoing and easy to understand national campaigns on cancer suspect symptoms should be prioritized and clinicians should have increased awareness of patients with leukemia‐suspicious symptoms or cytopenias to prevent delayed diagnosis. Future research should focus on the underlying mechanisms creating inequality in MDS outcomes and reasons for not allocating potential transplant candidates to allo‐HSCT should be clarified.
This study has several strengths. We conducted a population‐based cohort study 2233 patients unselected MDS patients using validated and detailed individual‐level data ascertained from routinely collected high‐quality Danish registries. The study was limited by lack of data on lifestyle factors, for example, smoking, which may have introduced unmeasured confounding. We further lacked data on mutational status and had some missing data on the IPSS and the IPSS‐R risk scores. Further, due to imprecise effect‐estimates, the potential association between SEP and the risk of progression to AML should be examined in a larger study population.
5. CONCLUSION
In conclusion, short education, living alone, and low income are adverse prognostic factors in MDS patients. This was not explained by difference in time to progression to AML, but may partly be explained by delayed diagnosis and lower transplantation rate among patients with lower SEP.
AUTHOR CONTRIBUTIONS
Tine Bichel Lauritsen, Lene Sofie Granfeldt Østgård, Kirsten Grønbæk, Jan Maxwell Nørgaard and Susanne Oksbjerg Dalton conceived of and designed the study. Tine Bichel Lauritsen conducted the analyses. Tine Bichel Lauritsen, Lene Sofie Granfeldt Østgård, Kirsten Grønbæk, Jan Maxwell Nørgaard and Susanne Oksbjerg Dalton interpreted the data and wrote the manuscript.
CONFLICT OF INTEREST
Authors have no conflicts of interest to declare.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/hon.3068.
Supporting information
Supplementary Material S1
ACKNOWLEDGMENTS
This study was supported by grants from The Danish Cancer Society (Kræftens Bekæmpelse, grant number R223‐A13094‐18‐S68), The Danish Research Center for Equality in Cancer (COMPAS), the Dagmar Marshalls Foundation, and the Einer Willumsens Mindelegat. Kirsten Grønbæk is supported by grants from The Danish Cancer Society (Kræftens Bekæmpelse, grant no. R223‐A13071, the Danish Research Center for Precision Medicine in Blood Cancers).
Lauritsen TB, Østgård LSG, Grønbæk K, Dalton SO, Nørgaard JM. Socioeconomic position and clinical outcomes in patients with myelodysplastic syndromes: a population‐based cohort study. Hematol Oncol. 2022;40(5):1056‐1066. 10.1002/hon.3068
DATA AVAILABILITY STATEMENT
Our institutional approval to use the data sources for the current study do not allow us to distribute or make patient data directly available to other parties. Interested researchers may apply for data access through the Research Service at the Danish Health Data Authority (e‐mail: forskerservice@sundhedsdata.dk; phone: +45 3268 5116). Up‐to‐date information on data access is available online (http://sundhedsdatastyrelsen.dk/da/forskerservice). Access to data from the Danish Health Data Authority requires approval from the Danish Data Protection Agency (https://www.datatilsynet.dk/english/the‐danish‐data‐protection‐agency/introduction‐to‐the‐danish[1]data‐protection‐agency/).
REFERENCES
- 1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391‐2405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 2. Moreno Berggren D, Folkvaljon Y, Engvall M, et al. Prognostic scoring systems for myelodysplastic syndromes (MDS) in a population‐based setting: a report from the Swedish MDS register. Br J Haematol. 2018;181(5):614‐627. 10.1111/bjh.15243 [DOI] [PubMed] [Google Scholar]
- 3. Dalton SO, Steding‐Jessen M, Jakobsen E, et al. Socioeconomic position and survival after lung cancer: influence of stage, treatment and comorbidity among Danish patients with lung cancer diagnosed in 2004–2010. Acta Oncol. 2015;54(5):797‐804. 10.3109/0284186x.2014.1001037 [DOI] [PubMed] [Google Scholar]
- 4. Ibfelt EH, Kjær SK, Høgdall C, et al. Socioeconomic position and survival after cervical cancer: influence of cancer stage, comorbidity and smoking among Danish women diagnosed between 2005 and 2010. Br J Cancer. 2013;109(9):2489‐2495. 10.1038/bjc.2013.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frederiksen BL, Dalton SO, Osler M, Steding‐Jessen M, de Nully Brown P. Socioeconomic position, treatment, and survival of non‐Hodgkin lymphoma in Denmark–a nationwide study. Br J Cancer. 2012;106(5):988‐995. 10.1038/bjc.2012.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Østgård LSG, Nørgaard M, Medeiros BC, et al. Effects of education and income on treatment and outcome in patients with acute myeloid leukemia in a tax‐supported health care system: a national population‐based cohort study. J Clin Oncol. 2017;35(32):3678‐3687. 10.1200/jco.2017.73.6728 [DOI] [PubMed] [Google Scholar]
- 7. Ibfelt EH, Dalton SO, Høgdall C, et al. Do stage of disease, comorbidity or access to treatment explain socioeconomic differences in survival after ovarian cancer? – a cohort study among Danish women diagnosed 2005–2010. Cancer Epidemiol. 2015;39(3):353‐359. 10.1016/j.canep.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 8. Dalton SO, Olsen MH, Johansen C, Olsen JH, Andersen KK. Socioeconomic inequality in cancer survival – changes over time. A population‐based study, Denmark, 1987–2013. Acta Oncol 2019;58(5):737‐744. 10.1080/0284186x.2019.1566772 [DOI] [PubMed] [Google Scholar]
- 9. Goldstein JS, Nastoupil LJ, Han X, Jemal A, Ward E, Flowers CR. Disparities in survival by insurance status in follicular lymphoma. Blood. 2018;132(11):1159‐1166. 10.1182/blood-2018-03-839035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kristinsson SY, Derolf AR, Edgren G, Dickman PW, Björkholm M. Socioeconomic differences in patient survival are increasing for acute myeloid leukemia and multiple myeloma in Sweden. J Clin Oncol. 2009;27(12):2073‐2080. 10.1200/jco.2008.18.2006 [DOI] [PubMed] [Google Scholar]
- 11. Larfors G, Moreno Berggren D, Garelius H, et al. Income, education and their impact on treatments and survival in patients with myelodysplastic syndromes. Eur J Haematol. 2021;107(2):219‐228. 10.1111/ejh.13641 [DOI] [PubMed] [Google Scholar]
- 12. Wang R, Gross CP, Halene S, Ma X. Neighborhood socioeconomic status influences the survival of elderly patients with myelodysplastic syndromes in the United States. Cancer Causes Control. 2009;20(8):1369‐1376. 10.1007/s10552-009-9362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. England JT, Zhang L, Buckstein R, et al. Income and outcome in myelodysplastic syndrome: the prognostic impact of SES in a single‐payer system. Leuk Res. 2013;37(11):1495‐1501. 10.1016/j.leukres.2013.08.021 [DOI] [PubMed] [Google Scholar]
- 14. Mastaglio F, Bedair K, Papaemmanuil E, et al. Impact of socioeconomic status on disease phenotype, genomic landscape and outcomes in myelodysplastic syndromes. Br J Haematol. 2016;174(2):227‐234. 10.1111/bjh.14042 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563‐591. 10.2147/clep.s179083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541‐549. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 17. Østgård LS, Nørgaard JM, Raaschou‐Jensen KK, et al. The Danish national acute leukemia Registry. Clin Epidemiol. 2016;8:553‐560. 10.2147/clep.s99460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lauritsen TB, Nørgaard JM, Grønbæk K, et al. The Danish myelodysplastic syndromes database: patient characteristics and validity of data records. Clin Epidemiol. 2021;13:439‐451. 10.2147/clep.s306857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Statistics Denmark [cited 2020 02.12] . Available from: https://www.statbank.dk/statbank5a/default.asp?w=1366
- 20. Jensen VM, Rasmussen AW. Danish education registers. Scand J Publ Health. 2011;39(7 Suppl l):91‐94. 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 21. Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Publ Health. 2011;39(7 Suppl l):103‐105. 10.1177/1403494811405098 [DOI] [PubMed] [Google Scholar]
- 22. United Nations Educational SaCOU . International Standard Classification of Education (ISCED 2011). 2012.
- 23. The Danish Transfusion Database [cited 2020 30.11] . Available from: https://www.rkkp.dk/om‐rkkp/de‐kliniske‐kvalitetsdatabaser/transfusionsdatabase/
- 24. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. 10.2147/clep.s91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lauritsen TB, Østgård LS, Grønbæk K, Dalton S, Jan Maxwell MN. Positive Predictive Values of Hematological Procedure Codes in the Danish National Patient Registry – a Population‐Based Validation Study. Pharmacoepidemiology and Drug Safety. 2022. (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ostgård LS, Nørgaard JM, Severinsen MT, et al. Data quality in the Danish national acute leukemia Registry: a hematological data resource. Clin Epidemiol. 2013;5:335‐344. 10.2147/clep.s48411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676‐682. 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 28. Lash Tjv TL, Haneuse S, Kenneth J, Rothman KJ. Modern Epidemiology. 4th ed. Lippincott Williams & Wilkens. 2021. [Google Scholar]
- 29. Platzbecker U, Fenaux P, Adès L, et al. Proposals for revised IWG 2018 hematological response criteria in patients with MDS included in clinical trials. Blood. 2019;133(10):1020‐1030. 10.1182/blood-2018-06-857102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersen PK, Perme MP. Pseudo‐observations in survival analysis. Stat Methods Med Res. 2010;19(1):71‐99. 10.1177/0962280209105020 [DOI] [PubMed] [Google Scholar]
- 31. Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull. 2007;81‐82(1):21‐37. 10.1093/bmb/ldm001 [DOI] [PubMed] [Google Scholar]
- 32. Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, Wolfe CD. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. 2015;14(12):1206‐1218. 10.1016/s1474-4422(15)00200-8 [DOI] [PubMed] [Google Scholar]
- 33. Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position (part 1). J Epidemiol Community Health. 2006;60(1):7‐12. 10.1136/jech.2004.023531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294(22):2879‐2888. 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Data Availability Statement
Our institutional approval to use the data sources for the current study do not allow us to distribute or make patient data directly available to other parties. Interested researchers may apply for data access through the Research Service at the Danish Health Data Authority (e‐mail: forskerservice@sundhedsdata.dk; phone: +45 3268 5116). Up‐to‐date information on data access is available online (http://sundhedsdatastyrelsen.dk/da/forskerservice). Access to data from the Danish Health Data Authority requires approval from the Danish Data Protection Agency (https://www.datatilsynet.dk/english/the‐danish‐data‐protection‐agency/introduction‐to‐the‐danish[1]data‐protection‐agency/).


