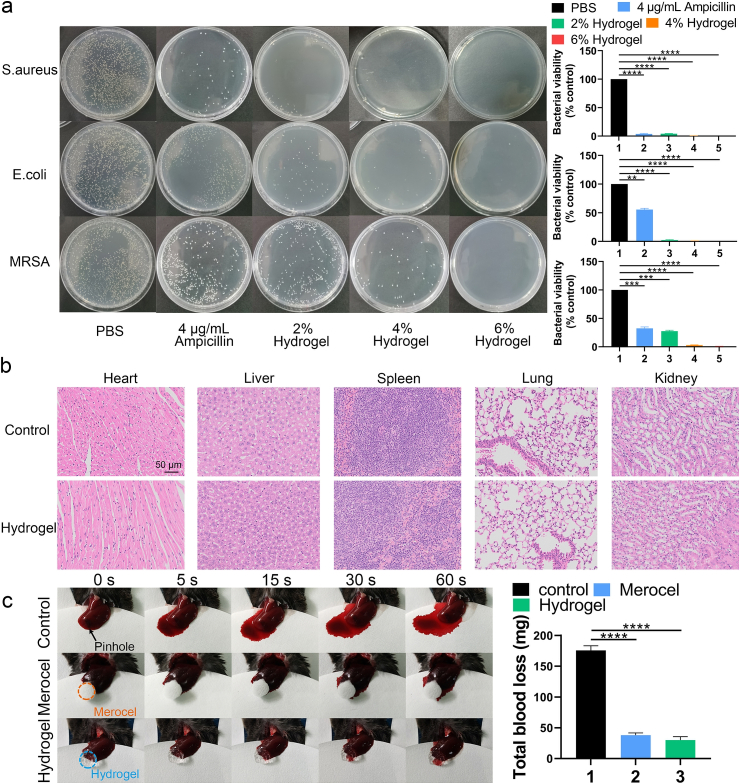
Fig. 2.
Analysis of the antibacterial function, biocompatibility and blood clotting ability of the HA-based hydrogel. (a) Bacterial clones and bacteria viability % of S. aureus, E. coli and MRSA after planting and incubation for 18 h at 37 °C. (b) H&E staining of major organs (heart, liver, spleen, lung and kidney) after administration of PBS or the HA hydrogel for 14 days (scale bar = 50 μm). (c) Hemostatic assay using the hemorrhaging liver mouse model. Data are presented as mean ± SD of triplicate experiments, **P < .01, ***P < .001, ****P < .0001.