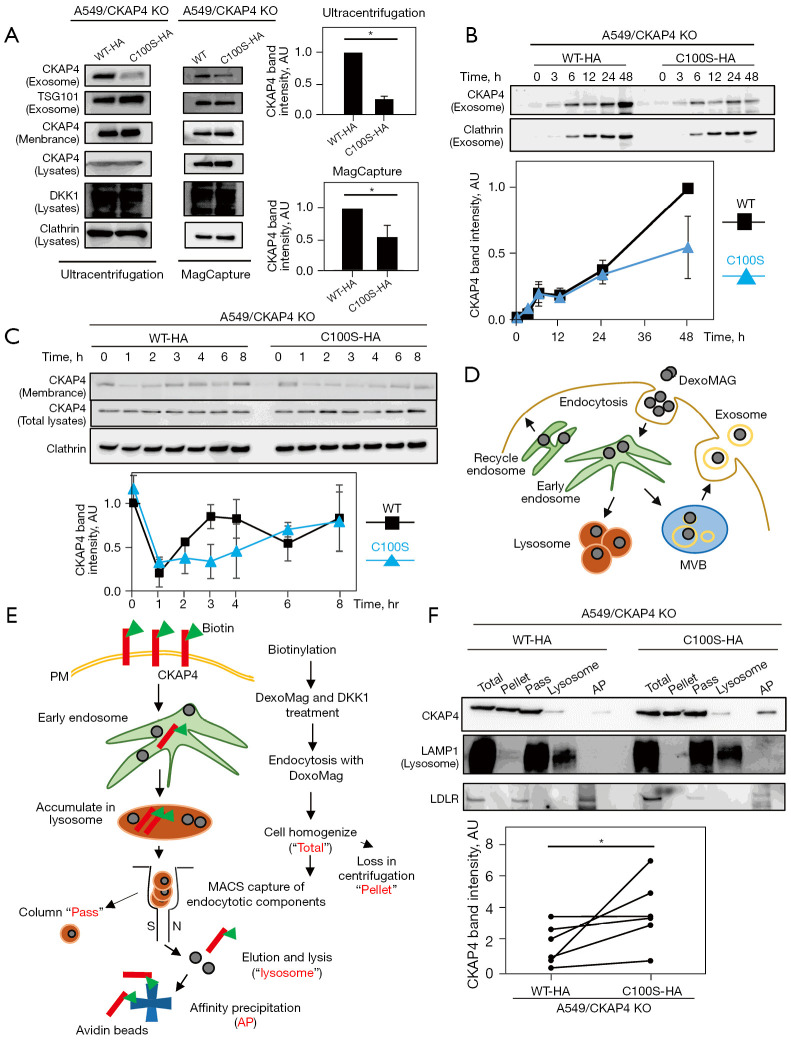
Figure 3.
CKAP4 palmitoylation is required for CKAP4 secretion with exosomes. (A) Left panels: SEVs were prepared by 100,000 ×g centrifugation or a MagCapture from the CM of A549/CKAP4 KO/CKAP4WT-HA (WT-HA) and A549/CKAP4 KO/CKAP4C100S-HA (C100S-HA) cells. Right panels: the band intensities of CKAP4 in exosomes were quantified. (B) Top panels: after medium change, A549/WT-HA and C100S-HA cells were incubated for indicated times. SEVs were prepared by 100,000 ×g centrifugation from the CM at each time period. Bottom panel: quantified CKAP4 band intensities at each time point were expressed as AU (the A549/WT-HA CKAP4 values at 48 h are 1). (C) Top panels: A549/WT-HA and A549/C100S-HA cells were stimulated with purified DKK1-FLAG proteins (250 ng/mL) for the indicated times. At different time points, cell surface proteins were biotinylated and precipitated. Bottom panel: quantified CKAP4 band intensities at time points were expressed as AU (A549/WT-HA values at 0 h are 1). (D) General schema for magnetically labeling of subcellular compartments along the endocytotic system using dextran-coated ferrofluid (DexoMAG) is shown. DexoMAG particles were endocytosed and accumulated in cellular endosomal compartments and the lysosome. (E) Schematic outline of workflow to separate the internalized PM proteins from subcellular compartments using MACS technology is shown. Cell surface proteins are biotinylated and cells were incubated with DKK1 CM containing DexoMAG and bafilomycin for 48 h. Cells were homogenized and organelles containing DexoMAG were magnetically captured and isolated. After elution, organelles were lysed and biotinylated proteins were precipitated with NeutrAvidin beads. Samples in red letters were subjected to immunoblot analysis in (F). (F) Top panels: subcellular compartments in A549/WT-HA or A549/C100S-HA cells along the endocytotic system were fractionated by MACS. Biotinylated cell surface proteins were affinity-precipitated with streptavidin beads from lysosomal fractions (AP). Total proteins, centrifuged precipitations after homogenization (pellets), MACS column flow-through proteins (pass), lysosomal proteins (lysosome), and APs were immunoblotted with indicated antibodies. Bottom panels: the band intensities of CKAP4 in the AP lanes (normalized by lysosome lanes) in six independent assays are quantified. Results are presented as the mean ± SD (A and C) or SE (B) of four independent experiments (excepting F). *, P<0.05 (Wilcoxon’s rank-sum test) (A and F). KO, knockout; WT-HA, wild-type CKAP4 with HA tag; C100S-HA, CKAP4C100S mutant with HA tag; AU, arbitrary unit; MVB, multivesicular body; PM, plasma membrane; MACS, magnetic activated cell sorting system; AP, affinity precipitation; SEVs, small extracellular vesicles; CM, conditioned medium.