Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by B‐cell hyperactivity and breach of tolerance. Autoreactive memory B cells, which have a decreased activation threshold and the ability to survive in absence of antigen, are believed to contribute to chronicity in autoimmune diseases like SLE. Belimumab, the first approved biological treatment of active SLE and lupus nephritis, reduces B cells dependent on B‐lymphocyte stimulator protein (BLyS) for survival, whereas memory B cells are spared; several studies reported circulating memory B‐cell concentrations increase following BLyS neutralization. This analysis investigated the effect of dose, demographics, and disease status on memory B‐cell response after starting belimumab treatment. Population pharmacodynamic models were fitted to a pooled dataset from seven belimumab SLE trials. The optimal model was selected using maximum likelihood methods and was then refit to the data using Bayesian analysis and used to simulate memory B‐cell response by belimumab dose and covariate subgroups. At the belimumab approved doses (10 mg/kg intravenously every 4 weeks, 200 mg subcutaneously every week), circulatory memory B cells increase in the first 4–8 weeks after belimumab initiation, typically returning to baseline levels over 76 weeks. The model analysis suggested belimumab stimulates memory B‐cell transition from lymphoid and/or inflamed tissues into the circulation, rather than inhibiting trafficking in the reverse direction. Baseline BLyS and anti–double‐stranded deoxyribonucleic acid antibody concentrations were statistically identifiable covariates of memory B‐cell response, although their impact on predicting size and response duration was small.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Memory B cells are believed to play an important role in systemic lupus erythematosus (SLE) pathogenesis. Belimumab treatment appears to spare memory B cells via B‐lymphocyte stimulator (BLyS) neutralization and initially increase circulating memory B‐cell levels.
WHAT QUESTION DID THIS STUDY ADDRESS?
What is belimumab's mode of action for increasing circulating memory B‐cell levels, and what are the effects of dose, demographics, and disease characteristics on this response?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Memory B‐cell response was predicted to be the same for approved belimumab doses (10 mg/kg intravenously every 4 weeks and 200 mg subcutaneously weekly). Modeling suggested that belimumab stimulates memory B‐cell transition from lymphoid and/or inflamed tissues into circulation. Elevated baseline levels of BLyS and anti–dsDNA antibodies were statistically identifiable covariates of memory B‐cell response, but their impact on predicting response size and duration was negligible.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
Understanding the rise in circulating memory B cells following belimumab treatment could offer adjunctive therapeutic targeting to improve outcomes for patients with SLE.
INTRODUCTION
The B‐lymphocyte stimulator protein (BLyS), also known as B‐cell activating factor, is a cytokine that plays an important role in the generation and differentiation of B cells. 1 Circulating levels of BLyS have been shown to be elevated in systemic lupus erythematosus (SLE) 1 , 2 , 3 and other autoimmune diseases 4 , 5 ; elevated BLyS is associated with disease activity. 6 , 7
Belimumab, an immunoglobulin G monoclonal antibody that binds to and inhibits the activity of BLyS, has been approved as a treatment for SLE and lupus nephritis in adults, and for SLE in children (≥5 years of age). 8 , 9 Belimumab is administered in adults or children (≥5 years of age) with SLE at a dose of 10 mg/kg intravenously (i.v.) every 4 weeks (q4w), or in adults with SLE at 200 mg subcutaneously (s.c.) every week (qw). 9
Following administration of belimumab, circulating levels of a broad range of B‐cell subsets are reduced due to the inhibition of BLyS activity and the subsequent downregulation of B‐cell synthesis. 10 , 11 The exceptions to this trend are memory B cells, which consistently show a rapid and significant increase in circulating levels in response to starting belimumab, followed by a gradual return to baseline levels over 76 weeks. 10 , 11 Memory B cells play an important role in the pathogenesis of SLE, resulting in the dysregulation of the immune system. 12 Furthermore, elevated levels of BLyS and memory B cells are associated with relapse in patients with SLE after B‐cell–depleting therapies. 13 Thus, it is informative to evaluate the memory B‐cell response to belimumab in patients with SLE. The reason for the initial increase in circulating memory B cells is not well‐understood but likely arises from a disruption to lymphocyte trafficking as opposed to a proliferative response. 14 This may be due to a redistribution of memory B cells from the lymphoid tissues (e.g., the spleen) and/or inflamed tissues into circulation, or due to an accumulation of circulating memory B cells if these cells are prevented from entering the tissues. 10 Upon repeat belimumab dosing and following the initial rise in circulating memory B‐cell levels, the reduction back to baseline is possibly a consequence of the limited lifespan of accumulated memory B cells coupled with a reduction in precursors of memory B cells, such as naïve B cells. 10 However, it cannot be excluded that some of the memory B cells gradually lose their cell markers used to identify this B‐cell subset when differentiating into plasma cells and thus could also lead to a reduction in the absolute number of memory B cells. 15 Irrespective of the exact mechanism by which belimumab increases memory B cells in circulation (mobilization out of, or prevention from entry into, lymphoid and/or inflamed tissues), memory B cells that have accumulated in circulation are destined for tissues. 16 Thus, disrupting their fate following belimumab administration may achieve adequate biological impact, as they can no longer effectively interact with other cells in an organized and structured manner that would be expected if resident within lymphoid or inflamed tissues. 17 Furthermore, because memory B cells appear to be spared from the depleting effects of BLyS neutralization, there may be potential benefit of targeting increased levels of circulating memory B cells post‐belimumab using an additional therapy such as an anti–CD20‐depleting agent. 3 , 10
The aim of this model‐based analysis is to characterize the memory B‐cell response upon starting belimumab treatment, to explore whether the mode of action can be inferred from the data and whether variability in response can be explained by dose, individual demographic information, or disease characteristics.
METHODS
Datasets
The analysis was based on a pooled dataset from patients with SLE across three phase II studies (GSK studies LBL02 [NCT00071487], 18 BEL112232 [NCT00732940], 19 and BEL114055 [PLUTO; NCT01649765] 20 ), three phase III studies (GSK studies BEL112341 [BLISS‐SC; NCT01484496], 21 , 22 BEL113750 [NCT01345253], 22 and BEL110751 [BLISS‐76; NCT00410384] 23 ), and one phase III/IV study (GSK study BEL115471 [EMBRACE; NCT01632241] 24 ). Belimumab was administered i.v. or s.c. in a double‐blind manner, except in BEL112232, which was an open‐label study of different s.c. regimens (Figure 1). All patients were starting belimumab treatment for the first time. All studies were ethics committee or institutional review board approved.
FIGURE 1.
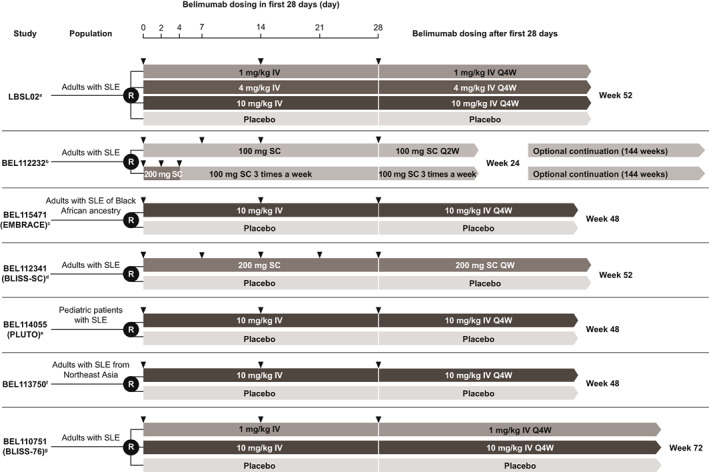
Studies providing memory B‐cell data. aWallace et al. 2009 18 ; b clinicaltrials.gov. 2013 19 ; cGinzler et al. 2021 24 ; 1924; dStohl et al. 2017 21 ; eBrunner et al. 2020 20 ; fZhang et al. 2018 22 ; gFurie et al. 2011. 23 IV, intravenous; Q2W, every 2 weeks; Q4W, every 4 weeks; QW, weekly; R, randomization; SC, subcutaneous; SLE, systemic lupus erythematosus.
Memory B‐cell quantification by flow cytometry
Memory B cells were quantified in blood by flow cytometry using the cell surface marker: CD19+ CD20+ CD27+, considered to define the total set of memory B cells, which are further divided into unswitched (CD19+ CD27+ IgDlo) and switched (CD19+ CD27+ IgD−) subtypes. 25 This model analysis was based on total CD19+ CD20+ CD27+ memory B‐cell levels.
Model structure
A population pharmacokinetic–pharmacodynamic (PK–PD) model was fitted to the memory B‐cell dataset. The belimumab dose was linked to the memory B‐cell response using an integrated PK–PD modeling approach. A patient's dosing history was converted into a belimumab concentration–time profile (PK component), which was used as the input to drive the memory B‐cell response (PD component).
Pharmacokinetics
Belimumab PK in patients with SLE follows two‐compartmental kinetics with first‐order s.c. absorption, distribution, and elimination. 26 , 27 PK data were not available for all patients, and individual belimumab exposure data were not included in the dataset for this analysis. Body weight and body mass index (BMI) have been identified as important patient level covariates of belimumab PK, describing the allometric effects of body size on PK. 26 , 27 The i.v. and s.c. PK profiles were derived for each patient in the analysis dataset using the population median PK parameters previously derived for patients with SLE, but taking into account between‐patient variability explained by body weight and BMI (Table S1). Pediatric patient data did not inform these adult i.v. and s.c. population PK models, but the PK in pediatric patients has been shown to be consistent with adults. 28
Memory B‐cell model
In the memory B‐cell model with peripheral compartment (Figure 2), compartment M represents memory B cells in circulation, and peripheral compartment P is unobserved and represents a pool of memory B cells outside of circulation in the lymphoid organs and/or tissues. Two potential mechanisms of action were considered. In Mechanism 1, the drug stimulus (ST[CBEL]) is a function of belimumab concentration in serum (C BEL) and drives the initial rise in memory B cells seen upon starting treatment (Figure 2a). The separate stimulus function (STP[C BEL]) reduces the amount in the peripheral compartment, which subsequently acts to reduce the circulating memory B‐cell input rate to deliver the return to baseline in circulating levels upon repeat dosing. Both stimulatory functions are modeled as saturable maximum effect (E max) functions, with maximum stimulus effects of E max and E maxP, and with half‐maximum effect achieved for the belimumab serum concentration (EC50). In Mechanism 2, inhibitory function (I[C BEL]) reduces the memory B‐cell removal rate from circulation, resulting in increased circulating levels upon starting belimumab treatment (Figure 2b). The separate inhibitory function (I P[C BEL]) reduces the peripheral input rate, and therefore the peripheral amount, resulting in circulating memory B cells returning to baseline upon repeat dosing. Both inhibitory functions are modeled as saturable inhibition expressions with maximum inhibition of I max and I maxP, with half‐maximal effect achieved at the belimumab serum concentration (IC50).
FIGURE 2.
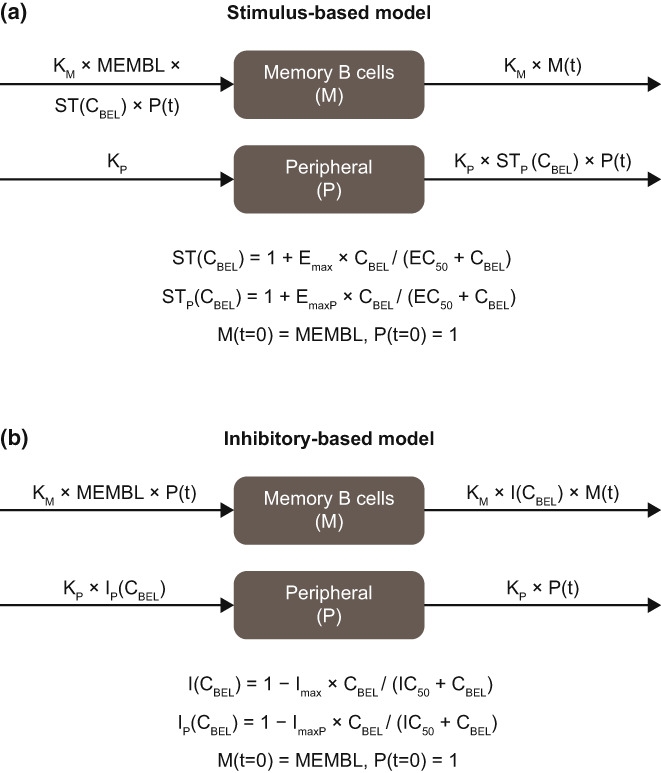
Drug stimulus effect (a) and drug inhibitory effect (b) models. EC50, belimumab serum concentration achieving half‐maximal response for the stimulatory function; E max, maximum response of stimulatory function; IC50, belimumab serum concentration achieving half‐maximal inhibition; C BEL, belimumab concentration in serum (μg/mL); I(C BEL), inhibitory function; I max, maximum response of inhibitory function; I P(C BEL), peripheral inhibitory function; K M, memory B‐cell removal rate; K P, memory B‐cell peripheral compartment removal rate; M, memory B cells in circulation; MEMBL, baseline memory B‐cell concentration in circulation; P, peripheral memory B cell; ST(C BEL), stimulatory function; STP(C BEL), peripheral stimulatory function; t, time after first belimumab dose.
Random effects
To capture between‐patient variability in the memory B‐cell response, model parameters of the memory B‐cell model were taken to be log‐normally distributed across the population: Parameter = θ × exp(η), where θ is the typical value and η is a normally distributed random variable. Maximum inhibition parameters (I max and I maxP) were instead modeled using the logistic function to constrain values between 0 and 1: Parameter = exp(θ + η)/(1 + exp(θ + η)). Where data were not sufficient to inform between‐patient variability for a model parameter, the parameter was modeled without random variability (η was set to zero). Residual variability on the observations was modeled using the exponential model: y = f × exp(ε), where y is the observed memory B‐cell concentration, f is the corresponding model predicted value, and ε a normally distributed random variable.
Model development and selection
A two‐step approach was used: maximum likelihood to explore several possible model solutions to identify the most appropriate model to describe memory B‐cell dynamics, followed by Bayesian sampling of the final model parameters to accurately characterize the precision in their estimates.
Maximum likelihood method
Model development was performed using the stochastic approximation expectation maximization (SAEM) algorithm in NONlinear Mixed Effects Modeling (NONMEM; version 7.3). The first stage involves a rapid approximation of individual parameter values using two samples per individual to iteratively converge toward the likely solution. In the second stage, individual parameter estimates are combined from previous iterations leading to population parameters converging toward the maximum likelihood solution. Model selection was primarily based on the objective function value and key visual predictive check (VPC) plots to confirm the model represented the data.
Covariate analysis
Covariates of potential interest were explored to see whether individual values explained any component of between‐patient variability and included demographic information (body weight, BMI, and age), baseline disease characteristics (BLyS, anti–double‐stranded deoxyribonucleic acid [anti‐dsDNA] antibody, complement proteins 3 and 4 [C3 and C4], and Safety of Estrogens in Lupus Erythematosus National Assessment—Systemic Lupus Erythematosus Disease Activity Index [SELENA‐SLEDAI] score). Using the individual predicted model parameters derived from the population model without covariates on the memory B‐cell model parameters, the hypothetical memory B‐cell response was simulated for each patient in the dataset for the same dose; belimumab 200 mg s.c. administered qw. In this way, the simulated memory B‐cell response was standardized for all patients, regardless of whether the patient received i.v. or s.c. belimumab in their respective studies, and enabled all patients to be combined into a single covariate analysis. Across all individuals, the simulated response at week 8 (memory B‐cell fold‐change from baseline) was compared with their observed covariate values in a linear regression. Under the null hypothesis of no correlation between the covariate and memory B‐cell response, any covariate‐parameter pair with p value less than 0.01 was considered potentially significant and included in the memory B‐cell response model. The updated model with additional covariates was refitted to the data and the relevance of each covariate assessed using the full covariate method. 29 For each covariate‐parameter pair, the ratio of the model parameter at the 2.5th and 97.5th percentiles in the covariate distribution was calculated with respect to the model parameter at the median covariate value. If the 2.5th or 97.5th percentile point estimates for the ratio were outside the 0.8–1.25 range, the covariate was considered a statistically identifiable covariate of memory B‐cell response.
Markov Chain Monte‐Carlo Bayesian method
The final model for memory B‐cell response was refit to the data using a fully Bayesian analysis implemented with Stan 30 , 31 and Torsten. 32 Stan's No U‐Turn Sampler algorithm, 33 an adaptive Hamiltonian Monte Carlo sampler, was used to generate samples from the joint posterior distribution of the model parameters. Weakly to moderately informative prior distributions were used for all parameters (see Table 2 in the Results). This was computationally challenging due to the large number of patients (N = 1880) who received belimumab, long observation periods (up to 76 weeks), and the need for a numerical solution of the model differential equations. A preliminary estimate of the elapsed time required to run one Hamiltonian Monte Carlo chain on a single processor was in the order of months. The time required to perform the analysis was reduced to ~1 week by using a within‐chain parallel computation method implemented in Torsten's group integrator function that distributes numerical integration across a grid using Message Passing Interface. 34
TABLE 2.
Final model parameter summary: Bayesian model estimates of model D
| Parameter | Fitted estimates and prior distributions | |
|---|---|---|
| Posterior median (SD) [90% CI] prior distribution | Between‐patient variability Log‐scale SD posterior median (SD) [90% CI] prior distribution | |
| EC50 (μg/ml) | 2.44 (0.818) [1.34, 4.04] | 0 |
| E max | 2.11 (0.114) [1.94, 2.32] | 0.696 (0.0379) [0.632, 0.756] a |
| × (Anti‐dsDNA/79) θ | 0.0535 (0.0166) [0.0261, 0.0806] | – |
| E maxP | 2.91 (0.251) [2.55, 3.37] | 0.948 (0.0727) [0.827, 1.07] a |
| × (Anti‐dsDNA/79) θ | 0.192 (0.0319) [0.143, 0.248] | – |
| K M (day−1) | 0.0631 (0.0112) [0.0499, 0.0859] | 1.16 (0.093) [1.01, 1.33] a |
| × (BLyS/1.13) θ | −0.125 (0.0428) [−0.195, −0.0537] | – |
| × (Anti‐dsDNA/79) θ | −0.0756 (0.0375) [−0.138, −0.0152] | – |
| Ratio K P/K M | 0.0237 (0.00626) [0.0142, 0.0348] | – |
| MEMBL (cells/μl) | 14.7 (0.360) [14.1, 15.3] | 0.983 (0.0186) [0.953, 1.02] a |
| y = f × exp(ε) b | 0.348 (0.00413) [0.341, 0.355] | – |
Note: N(μ,σ), normal distribution with mean μ and SD σ; half‐N(0,σ), half‐normal distribution with SD σ; log‐N(μ,σ), log‐normal distribution with log‐scale mean μ and log‐scale SD σ (the μ and σ values chosen for the EC50 prior was chosen to be centered around 4 with 97.5% likelihood of being less than 10).
Abbreviations: BLyS, B‐lymphocyte stimulator protein; CI, confidence interval; anti‐dsDNA, anti‐double‐stranded deoxyribonucleic acid antibody; ε, normally distributed random variable for within patient variability; EC50, belimumab serum concentration achieving half‐maximal response for the stimulatory function; E max, maximum response of stimulatory function; E maxP, maximum response of memory B‐cell peripheral stimulatory function; f, model predicted memory B‐cell concentration; θ, fixed effect covariate parameter; K M, memory B‐cell removal rate; K P, memory B‐cell peripheral compartment removal rate; LKJ, Lewandowski‐Kurowicka‐Joe; MEMBL, memory B‐cell concentration at baseline; P, peripheral memory B cell; SD, standard deviation; SE, standard error; y, observed memory B‐cell concentration.
Between‐patient variability is characterized by a covariance matrix, with prior distribution of each SD term described by a half‐normal distribution, and the prior distribution of the correlation terms described by the LKJ (2) distribution. 35
Residual variability as characterized by the SD on the random variable ε.
Final model simulations
The final model was used to simulate the memory B‐cell response for the belimumab doses approved for SLE (10 mg/kg i.v. q4w and 200 mg s.c. qw) and for the lower dose tested in the phase III trial BEL110751 (1 mg/kg i.v. q4w). Further simulations explored the memory B‐cell response for different memory B‐cell baseline levels and for differences in any covariate included in the final model.
RESULTS
Dataset characteristics
The population analysis dataset contained 2753 patients with SLE: 1880 (68.3%) received i.v. or s.c. belimumab and 873 (31.7%) placebo (Figure S1). Of the belimumab‐treated patients, 1216 (64.7%) and 611 (32.5%) were adults who received belimumab i.v. and s.c., respectively, and the remaining 53 (2.8%) were children aged 5–17 years who received belimumab i.v. Baseline characteristics are summarized (Table S2). Observed memory B‐cell levels over time show a clear response in belimumab‐treated patients but not placebo (Figure S2).
Model development using SAEM in NONMEM
The model was fitted to the memory B‐cell data from all belimumab‐treated patients. Placebo patients were not included due to an absence of memory B‐cell response in these patients (Figure S2).
Parameter identifiability
Not all model parameters were uniquely identifiable from the data. Between‐patient variability was not included on the EC50 and IC50 parameters of the stimulatory and inhibitory functions, respectively. Early memory B‐cell response observed at week 8 versus individual predicted average concentration over the first 4 weeks showed evidence of an exposure‐response indicating the EC50 or IC50 could be identified, with an empirically derived mean (±standard error) of 7.3 (±2.6) μg/ml (Figure S3), used as a weakly informative prior to stabilize model fits during initial development in NONMEM. The memory B‐cell compartment removal rate (K M) was estimated with between‐patient variability, but the peripheral compartment removal rate (K P) was set proportional to K M (K P = θ × K M) and the ratio θ estimated from the data.
Stimulatory versus inhibitory model
Based on the objective function, the stimulatory function model (Model A) was a better fit to the data than the inhibitory function model (Model B; Table 1). Model A was taken forward for further development.
TABLE 1.
Initial model development using maximum likelihood method
| Model | Description | Objective function value | E max/I max estimates |
|---|---|---|---|
| Model A | Stimulatory response function | 46,923 |
E max = 2.66 E maxP = 2.87 |
| Model B | Inhibitory response function | 48,457 |
I max = 71.6% I maxP = 75.2% |
| Model C | Stimulatory response function with baseline covariates BLyS and anti‐dsDNA | 46,390 |
E max = 2.70 E maxP = 3.05 |
| Model D | Stimulatory response function with baseline covariates BLyS and anti‐dsDNA; reduced model | 46,484 |
E max = 2.91 E maxP = 3.26 |
Abbreviations: BLyS, B‐lymphocyte stimulator protein; anti‐dsDNA, anti‐double‐stranded deoxyribonucleic acid antibody; E max, maximum response of stimulatory function; E maxP, maximum response of memory B‐cell peripheral stimulatory function; I max, maximum response of inhibitory function; I maxP, maximum response of memory B‐cell peripheral inhibitory function.
Covariate analysis
The memory B‐cell response (fold‐change from baseline at week 8) was simulated for all belimumab‐treated patients in the analysis dataset using the individual predicted model parameter values for each patient from Model A (Table 1), assuming each patient received belimumab 200 mg s.c. qw (see Methods); the simulated memory B‐cell response at week 8 was plotted against the observed covariate values for each patient. Linear regression predicted baseline BLyS and baseline anti‐dsDNA concentrations were significantly correlated with memory B‐cell response (p value <0.01), although the correlations were weak (adjusted R 2 values of 0.036 for BLyS and 0.020 for anti‐dsDNA; Table S3). Baseline BLyS and baseline anti‐dsDNA levels were added as covariates to E max, E maxP, and K M (model parameters with between‐patient variability) and the model refitted to the data (Model C; Table 1). The full‐covariate method was then applied to assess the impact of each covariate (Figure 3a) and confirmed that baseline BLyS was a relevant covariate of K M (but not E max and E maxP), with higher BLyS levels associated with smaller memory B‐cell removal rates and therefore longer half‐lives. Baseline anti‐dsDNA was confirmed as a relevant covariate of the maximal response parameters, E max and E maxP, and also K M, with higher levels associated with a larger maximal response and lower K M.
FIGURE 3.
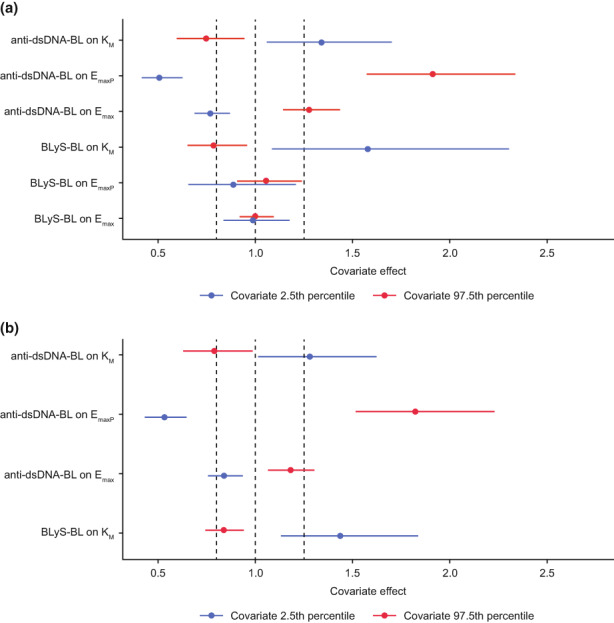
Full covariate method applied to stimulatory response model: (a) Model C; (b) Model D (the final model) inferred from the Bayesian model analysis. BLyS‐BL, B‐lymphocyte stimulator levels at baseline; anti‐dsDNA‐BL, anti‐double‐stranded deoxyribonucleic acid antibody levels at baseline; E max, maximum response of stimulatory function; E maxP, maximum response of stimulatory function of the peripheral compartment; K M, memory B‐cell removal rate. Influence of covariate on model parameter as a multiplicative effect: the vertical dotted line at x = 1.0 represents no effect; the dotted lines at 0.8 and 1.25 represent the interval over which the covariate effect is not considered pharmacokinetically relevant. Points represent the point estimate of the covariate effect at the 2.5th (blue) and 97.5th (red) percentile in the covariate distribution. The horizontal bars represent the 95% confidence interval due to the precision estimate in the corresponding model covariate parameter.
Final model selection (Model D)
The final model (Model D, Table 1) was selected to include only the statistically relevant covariate‐parameter pairs: baseline BLyS on K M and baseline anti‐dsDNA on E max, E maxP, and K M. Model simulations (belimumab 10 mg/kg i.v. q4w) for Model D show the memory B‐cell dynamics in circulation and in the unobserved peripheral compartment (Figure 4). The simulated memory B‐cell response in circulation shows the typical increase upon starting belimumab, followed by the slower return to baseline. The simulation also indicates a relatively slow depletion of the peripheral compartment, which matches the naïve B cell reduction observed in the phase III adult SLE study BEL110751 for the same dose (belimumab 10 mg/kg i.v. q4w). 10 , 23 All mature B cells start as naïve B cells before proliferating into various subtypes, one subtype being memory B cells. By interpreting the peripheral compartment as representing a pool of extra‐circulatory memory B cells, the reduction over time of this unobserved state could be expected to follow the fold reduction in naïve B‐cell levels. On this basis, Model D (the final model) is considered to represent the underlying memory B‐cell pharmacology and therefore the memory B‐cell response to starting belimumab treatment.
FIGURE 4.
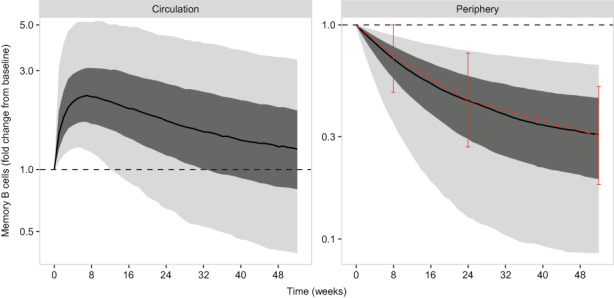
Simulation (Model D, final model) for 10 mg/kg i.v. dosing every 4 weeks. E max, maximum response of the model stimulatory function. Median (black line), interquartile range (dark gray region) and 90% prediction interval (light gray region) are shown. The median memory B‐cell level at baseline is shown as the horizontal black dotted line. The naive B‐cell fold change from baseline is superimposed on the peripheral memory B‐cell levels (that is, the unobserved memory B cells outside of circulation), as the median (red points) and interquartile range (solid vertical bars).
Final model summary (Model D)
The final model was fitted to the data using Stan augmented with the Torsten functions. Weakly to moderately informative prior distributions (normal, log‐normal or half‐normal distributions) were used for the fixed and random‐effect model parameters. The prior distribution for the correlation matrix for between‐patient variability was characterized by the Lewandowski‐Kurowicka‐Joe (LKJ) distribution 35 (Table 2).
Eight Markov Chain Monte Carlo (MCMC) chains were run with 250 warmup and 250 post‐warmup iterations per chain. Three of the chains were discarded due to sampling problems, leaving 1250 post‐warmup samples for parameter estimation and other model‐based inferences. Computations for each chain were distributed over 48 central processing units, and the elapsed time to complete the slowest chain was 4 days. Final parameters are shown (Table 2), and VPC plots show that the model fitted the observed data well (Figure S4). The full covariate method evaluated from the Bayesian sampled parameters confirms that baseline BLyS is a statistically identifiable covariate of K M, and baseline anti‐dsDNA a statistically identifiable covariate of E max, E maxP, and K M (Figure 3b); point estimates of the 2.5th and 97.5th percentiles of baseline anti‐dsDNA on E max are just within the 0.8 to 1.25 interval, but the confidence intervals extend outside this range.
Final model simulations (Model D)
The model was used to simulate various dosing regimens and for the approved s.c. dose (200 mg s.c. qw) stratified by baseline concentrations of memory B cells, BLyS, and anti‐dsDNA antibody (Figures 5 and S5).
FIGURE 5.
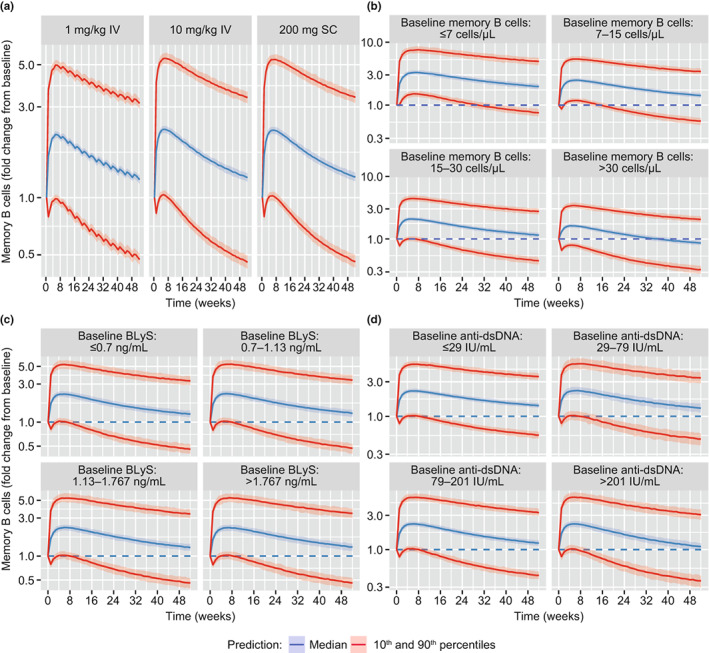
Simulated memory B‐cell response (fold‐change from baseline), (a) for i.v. and s.c. dosing regimens; (b) by quartiles of baseline memory B‐cell levels for 200 mg s.c. dosing; (c) by quartiles of baseline BLyS levels for 200 mg s.c. dosing; and (d) by quartiles of baseline anti‐dsDNA levels for 200 mg s.c. dosing. BLyS, B‐lymphocyte stimulator protein; anti‐dsDNA, anti‐double‐stranded deoxyribonucleic acid antibody; IV, intravenous; SC, subcutaneous. Shaded regions represent the 90% credible interval around the median (blue line) and 10th and 90th percentiles (red lines).
DISCUSSION
This model‐based analysis characterized the memory B‐cell response to belimumab treatment in patients with SLE, using data from previous belimumab clinical trials. Upon initiating belimumab therapy, circulating memory B cells rapidly increase before slowly returning to baseline (Figure S2). 10 , 11 The dynamics of this response were explored using population PK–PD modeling. The final model identified to best explain the data was refitted to the data using a fully Bayesian method implemented with Stan and Torsten, to provide a more rigorous characterization of the uncertainty in model parameters and predictions, and permitted probabilistic inferences about predicted B‐cell responses to belimumab treatment. Model exploration and selection leading up to that final model were performed using the SAEM method in NONMEM rather than the fully Bayesian method due to prohibitive time and computation costs for such Bayesian analyses.
The memory B‐cell response was predicted to be the same for either belimumab dosing regimens approved for SLE (10 mg/kg i.v. q4w and 200 mg s.c. qw, Figure 5a). The initial increase in memory B cells occurred over the first few weeks of treatment, typically achieving a two‐fold increase between weeks 4 and 8 (median [95% CI] fold‐change of 2.21 [2.13–2.32] by week 4 for 200 mg s.c. qw), before slowly returning to baseline after 76 weeks of treatment. The rapid increase over the first 4 weeks post‐belimumab initiation suggests the increase can be explained by memory B‐cell redistribution from lymphoid and inflamed tissues, rather than through increased proliferation. 14 The mechanism for this redistribution is unclear, but the model analysis favors a stimulatory, rather than inhibitory, mode of action; that is, belimumab acts to stimulate the transition of memory B cells from the lymphoid and/or inflamed tissues into the circulation.
Model simulations suggest the memory B‐cell response is rapid, with a substantial increase in circulating levels 1 week after starting belimumab 200 mg s.c. (median [95% CI] fold‐change from baseline of 1.67 [1.59–1.75]), consistent with a recent study where increased memory B‐cell levels were observed as early as 2 weeks after starting belimumab. 14 Similar results have been observed for other BLyS antagonists, specifically blisibimod, where increased memory B‐cell levels were also apparent by week 2. 36 After the first belimumab s.c. dose, serum concentrations reach ~25 μg/ml, 27 ~10‐fold higher than the EC50 for the memory B‐cell response (Table 2). On this basis, serum concentrations even after the first belimumab dose are sufficient to drive the memory B‐cell response. However, a stimulatory mechanism of action localized within lymphoid or inflamed tissues, which drives memory B cells into circulation within a week of dosing, would also require sufficient belimumab distribution into these peripheral sites over this period. Belimumab is a large molecule (147 kDa) and mainly restricted to systemic circulation with a low volume of distribution (5 L) compared with plasma volume (~3 L). 9 Nevertheless, there is some distribution into peripheral tissues—the distributional half‐life of belimumab is ~1.1 days, 27 and we speculate that belimumab distribution into lymphoid or inflamed tissues may contribute to a localized effect acting to stimulate redistribution of memory B cells out of these tissues and into circulation.
Owing to the variability in memory B‐cell response at the lower 1 mg/kg i.v. dose level in the population analysis dataset, the belimumab concentration to achieve half‐maximal response was identifiable. This was predicted to be 2.44 μg/ml (Table 2), much smaller than the average concentration at steady state for the approved i.v. and s.c. doses (~100 μg/ml). This result is consistent with model simulations, which predicted almost equivalent memory B‐cell response for belimumab 1 and 10 mg/kg i.v. q4w, and also for 200 mg s.c. qw (Figure 5a). The result confirms the response is saturated at 10 mg/kg i.v. or 200 mg s.c. dose levels approved for SLE, and a greater redistribution of memory B cells into circulation would not be observed at higher doses. The memory B‐cell response was predicted to be observed in all patients, regardless of baseline memory B‐cell level (Figure 5b). However, patients with high baseline levels were predicted to have a slightly smaller fold increase from baseline, and an earlier return to baseline, compared with those with low baseline levels.
Pharmacological measures of disease activity (baseline BLyS and anti‐dsDNA levels) were statistically identifiable covariates of memory B‐cell response (Figure 3). Higher baseline BLyS levels were associated with a lower memory B‐cell removal rate (K M), consistent with the role of BLyS in promoting B‐cell survival. Higher baseline anti‐dsDNA levels were associated with higher maximal response in memory B‐cell redistribution. Anti‐dsDNA levels correlate with disease activity; therefore this result suggests there is perhaps greater potential to respond to the effects of BLyS neutralization in patients with high disease activity. However, although BLyS and anti‐dsDNA were identified as statistically identifiable covariates of memory B‐cell response, their impact on predicting the size and duration of response was negligible relative to the overall variability (Figure 5c,d).
In summary, belimumab‐induced redistribution of memory B cells into circulation may be a consequence of disrupting the germinal center and extrafollicular structures associated in the generation of pathogenic autoreactivity in SLE, 37 and it can be hypothesized that this mechanism may be a contributing factor to belimumab efficacy in the treatment of SLE.
Model analysis has confirmed that, at the approved i.v. and s.c. belimumab doses, memory B‐cell redistribution is maximal in all patients; on the basis that redistribution contributes to efficacy, there is no evidence to suggest higher doses would offer greater benefit in this regard. Initiating belimumab therapy to increase memory B cells in circulation for targeting with an anti‐CD20+ agent (such as rituximab) may in theory achieve long‐term benefit if a greater number of autoreactive memory B cells are eliminated via this process. However, the model analysis was consistent with a relatively slow depletion in unobserved memory B cells outside of circulation (Figure 4). On this basis, even if all circulating cells were eliminated by an anti‐CD20+ agent, a significant pool of memory B cells outside circulation remain, and it is unclear to what extent anti‐CD20+ agents are able to effectively target memory B cells residing deep within lymphoid organs or inflamed tissues.
CONCLUSIONS
In patients with SLE receiving belimumab 10 mg/kg i.v. or 200 mg s.c., a two‐fold increase in circulating memory B‐cell levels is typically observed over the first 4–8 weeks after starting belimumab therapy, returning to baseline or lower levels over 76 weeks. Our modeling suggests that belimumab stimulates the transit of memory B‐cells from lymphoid and/or inflamed tissues into the circulation, rather than inhibiting trafficking in the opposite direction. This memory B‐cell response is expected to be observed in all patients regardless of baseline memory B‐cell levels and did not correlate strongly with any other individual demographic information or baseline disease status.
AUTHOR CONTRIBUTIONS
All authors designed the research. R.D. and A.V.M. performed the research. All authors analyzed the data and wrote the manuscript.
FUNDING INFORMATION
This study was funded by GSK (Studies LBSL02, BEL112232, BEL115471, BEL112341, BEL114055, BEL113750, and BEL117051).
CONFLICT OF INTEREST
R.D. and A.V.M. are employees of GSK and hold stocks and shares in the company. W.R.G. is an employee of Metrum Research Group, and his work on this project was funded by GSK.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This analysis was funded by GSK. The authors would like to thank the participating patients and their families, clinicians, and study investigators. Medical writing assistance was provided by Helen Taylor, PhD, Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.
Dimelow R, Gillespie WR, van Maurik A. Population model‐based analysis of the memory B‐cell response following belimumab therapy in the treatment of systemic lupus erythematosus. CPT Pharmacometrics Syst Pharmacol. 2023;12:462‐473. doi: 10.1002/psp4.12919
DATA AVAILABILITY STATEMENT
Anonymized individual patient data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
REFERENCES
- 1. Cancro MP, D'Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest. 2009;119:1066‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697‐1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teng YKO, Bruce IN, Diamond B, et al. Phase III, multicentre, randomised, double‐blind, placebo‐controlled, 104‐week study of subcutaneous belimumab administered in combination with rituximab in adults with systemic lupus erythematosus (SLE): BLISS‐BELIEVE study protocol. BMJ Open. 2019;9:e025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mariette X, Roux S, Zhang J, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren's syndrome. Ann Rheum Dis. 2003;62:168‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune‐based rheumatic diseases. Arthritis Rheum. 2001;44:1313‐1319. [DOI] [PubMed] [Google Scholar]
- 6. Petri M, Stohl W, Chatham W, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58:2453‐2459. [DOI] [PubMed] [Google Scholar]
- 7. Matsushita T, Hasegawa M, Yanaba K, Kodera M, Takehara K, Sato S. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum. 2006;54:192‐201. [DOI] [PubMed] [Google Scholar]
- 8. Baker KP, Edwards BM, Main SH, et al. Generation and characterization of LymphoStat‐B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48:3253‐3265. [DOI] [PubMed] [Google Scholar]
- 9. GSK . US highlights of prescribing information 2021.
- 10. Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64:2328‐2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Struemper H, Kurtinecz M, Edwards L, Freimuth WW, Roth DA, Stohl W. Reductions in circulating B cell subsets and immunoglobulin G levels with long‐term belimumab treatment in patients with SLE. Lupus Sci Med. 2022;9:e000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manz RA, Moser K, Burmester GR, Radbruch A, Hiepe F. Immunological memory stabilizing autoreactivity. Curr Top Microbiol Immunol. 2006;305:241‐257. [DOI] [PubMed] [Google Scholar]
- 13. Cambridge G, Isenberg DA, Edwards JC, et al. B cell depletion therapy in systemic lupus erythematosus: relationships among serum B lymphocyte stimulator levels, autoantibody profile and clinical response. Ann Rheum Dis. 2008;67:1011‐1016. [DOI] [PubMed] [Google Scholar]
- 14. Arends EJ, Zlei M, Tipton CM, et al. POS0680 belimumab add‐on therapy mobilises memory B cells into the circulation of patients with SLE. Ann Rheum Dis. 2021;80:585. [Google Scholar]
- 15. Jourdan M, Caraux A, Caron G, et al. Characterization of a transitional preplasmablast population in the process of human B cell to plasma cell differentiation. J Immunol. 2011;187:3931‐3941. [DOI] [PubMed] [Google Scholar]
- 16. Palm AE, Henry C. Remembrance of things past: long‐term B cell memory after infection and vaccination. Front Immunol. 2019;10:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117‐139. [DOI] [PubMed] [Google Scholar]
- 18. Wallace DJ, Stohl W, Furie RA, et al. A phase II, randomized, double‐blind, placebo‐controlled, dose‐ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 2009;61:1168‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. clinicaltrials.gov . Phase 2 study of belimumab administered subcutaneously to subjects with systemic lupus erythematosus (SLE) 2013. NCT00732940 .
- 20. Brunner HI, Abud‐Mendoza C, Viola DO, et al. Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo‐controlled trial. Ann Rheum Dis. 2020;79:1340‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stohl W, Schwarting A, Okada M, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty‐two‐week randomized, double‐blind, Placebo‐Controlled Study. Arthritis Rheumatol. 2017;69:1016‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang F, Bae SC, Bass D, et al. A pivotal phase III, randomised, placebo‐controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. 2018;77:355‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo‐controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918‐3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ginzler E, Guedes Barbosa LS, D'Cruz D, et al. Phase III/IV, randomized, fifty‐two‐week study of the efficacy and safety of belimumab in patients of black African ancestry with systemic lupus erythematosus. Arthritis Rheumatol. 2022;74:112‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanz I, Wei C, Jenks SA, et al. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front Immunol. 2019;10:2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Struemper H, Chen C, Cai W. Population pharmacokinetics of belimumab following intravenous administration in patients with systemic lupus erythematosus. J Clin Pharmacol. 2013;53:711‐720. [DOI] [PubMed] [Google Scholar]
- 27. Struemper H, Thapar M, Roth D. Population pharmacokinetic and pharmacodynamic analysis of belimumab administered subcutaneously in healthy volunteers and patients with systemic lupus erythematosus. Clin Pharmacokinet. 2018;57:717‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dimelow R, Ji B, Struemper H. Pharmacokinetics of belimumab in children with systemic lupus erythematosus. Clin Pharmacol Drug Dev. 2021;10:622‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gastonguay M. Full Covariate Models as an Alternative to Methods Relying on Statistical Significance for Inferences About Covariate Effects: A Review of Methodology and 42 Case Studies. Population Approach Group in Europe (PAGE) 2011;Abstr. 2229.
- 30. Carpenter B, Gelman A, Hoffman MD, et al. Stan: a probablistic programming language. J Stat Softw. 2017;76:1‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Margossian C, Gillespie WR. Stan functions for Bayesian pharmacometric modeling. J Pharmacokinet Pharmacodyn. 2016;43:S52. [Google Scholar]
- 32. Metrum Research Group . Torsten Version 0.89.0: A pharmacokinetics/pharmacodynamics library for Stan. https://metrumresearchgroup.github.io/Torsten/. Accessed November 24, 2021.
- 33. Hoffman MD, Gelman A. The no‐U‐turn sampler: adaptively setting path lengths in Hamiltonian Monte Carlo. J Mach Learn Res. 2011;15:1593‐1623. [Google Scholar]
- 34. MPI: a message‐passing interface standard version 3.1. High Performance Computing Center Stuttgart (HLRS); 2015.
- 35. Lewandowski D, Kurowicka D, Joe H. Generating random correlation matrices based on vines and extended onion method. J Multivar Anal. 2009;100:1989‐2001. [Google Scholar]
- 36. Stohl W, Merrill JT, Looney RJ, et al. Treatment of systemic lupus erythematosus patients with the BAFF antagonist "peptibody" blisibimod (AMG 623/A‐623): results from randomized, double‐blind phase 1a and phase 1b trials. Arthritis Res Ther. 2015;17:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jenks SA, Cashman KS, Woodruff MC, Lee FE, Sanz I. Extrafollicular responses in humans and SLE. Immunol Rev. 2019;288:136‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Anonymized individual patient data and study documents can be requested for further research from www.clinicalstudydatarequest.com.


