Abstract
Background/Aims:
Phthalates and their replacements are endocrine/metabolic disruptors that may impact gestational weight gain (GWG) – a pregnancy health indicator. We investigated overall and fetal sex-specific associations of individual and cumulative phthalate/replacement biomarkers with GWG.
Methods:
Illinois women (n=299) self-reported their weight pre-pregnancy and at their final obstetric appointment before delivery (median 38 weeks). We calculated pre-pregnancy body mass index and gestational age-specific GWG z-scores (GWGz). We quantified 19 phthalate/replacement metabolites (representing 10 parent compounds) in pools of up-to-five first-morning urine samples, collected approximately monthly between 8-40 weeks gestation. We used linear regression, quantile-based g-computation (QGComp), or weighted quantile sum regression (WQSR) to evaluate associations of ten biomarkers (individual metabolites or parent molar-sums) individually or as mixtures (in interquartile range intervals) with GWGz. We evaluated associations in all women and stratified by fetal sex.
Results:
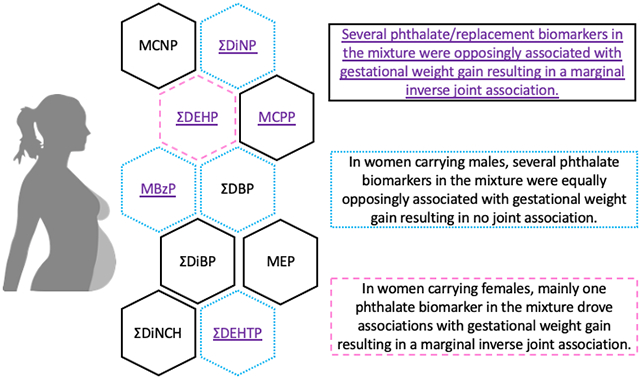
Individually, sums of metabolites of di(2-ethylhexyl) phthalate (ƩDEHP), di(isononyl) cyclohexane-1,2-dicarboxylate (ƩDiNCH), and di(2-ethylhexyl) terephthalate (ƩDEHTP) had consistent inverse associations with GWGz, and some associations were fetal sex-specific. When evaluating phthalates/replacements as a mixture, QGComp identified ƩDEHP, ƩDEHTP, and mono-(3-carboxypropyl) phthalate, along with sum of di(isononyl) phthalate metabolites (ƩDiNP) and monobenzyl phthalate as notable contributors to lower and higher GWGz, respectively, resulting in a marginal inverse joint association in all women (β: −0.29; 95% CI: −0.70, 0.12). In women carrying females, ƩDEHP contributed to marginal inverse joint association (β: −0.54; 95% CI: −1.09, 0.03). However, there was no overall association in women carrying males (β: 0.00; 95% CI: −0.60, 0.59), which was explained by approximately equal negative (driven by ƩDEHTP) and positive (driven by ƩDiNP) partial associations. WQSR analyses consistently replicated these findings.
Conclusions:
Biomarkers of phthalates/replacements were fetal sex-specifically associated with GWGz. Because DEHTP contributed substantively to mixture associations, additional studies in pregnant women may be needed around this plasticizer replacement.
Keywords: phthalates, DiNCH, DEHTP, gestational weight gain, pregnancy, fetal sex
Graphical Abstract
1. INTRODUCTION
Gestational weight gain (GWG) is an important, easily monitored indicator of maternal and fetal health. Deviations from the Institute of Medicine (IOM) GWG guidelines, including both excessive and inadequate GWG, may negatively impact health (Rasmussen and Yaktine, 2009). For example, compared to women with adequate GWG, those with inadequate GWG are at higher risk of delivering pre-term or small-for-gestational age newborns, whereas women with excessive GWG are at higher risk of developing gestational hypertension and having large-for-gestational age newborns (Li et al., 2013). Long-term risks of inappropriate GWG include increased likelihood of maternal postpartum depression (Zanardo et al., 2021), excessive offspring adiposity (Lau et al., 2014), and greater maternal postpartum weight retention, all of which could lead to increased risk of later life-associated health complications (Hutchins et al., 2020; Margerison Zilko et al., 2010). Therefore, it is critical to identify modifiable risk factors associated with inappropriate GWG.
Phthalates are a class of chemicals found in a wide variety of consumer products, including (but not limited to) food packaging materials, coatings of medications and supplements, personal care products, and cosmetics, with ubiquitous exposure among pregnant women in the U.S. (Woodruff et al., 2011). This is particularly concerning given that certain phthalates have been implicated in pregnancy-related metabolic disorders, including gestational hypertension (Cantonwine et al., 2016; Soomro et al., 2021; Werner et al., 2015) and diabetes (Fisher et al., 2018; James-Todd et al., 2018; Shaffer et al., 2019). Industry use of plasticizer replacements such as cyclohexane-1,2-dicarboxylic acid diisononyl ester (DiNCH, a non-phthalate alternative) and di(2-ethylhexyl) terephthalate (DEHTP) appear to be on the rise (Bui et al., 2016; Lemke et al., 2021; Lessmann et al., 2016). These replacements may have similar hormonally-mediated adverse health impacts as the original phthalates (Campioli et al., 2017; Fruh et al., 2021; Lee et al., 2020; Machtinger et al., 2018; Zhang et al., 2020). Pregnancy and fetal development, including GWG, are regulated by coordinated hormonal, inflammatory, and metabolic processes, which may be biological targets of phthalates/replacements (Gore et al., 2015; Maradonna and Carnevali, 2018; Mohammadi and Ashari, 2021).
Current evidence evaluating associations of prenatal urinary concentrations of phthalate metabolites (used as biomarkers of phthalate exposure) with GWG is inconclusive (Bellavia et al., 2017; Gao et al., 2021; James-Todd et al., 2016; Li et al., 2019a; Philips et al., 2020; Tyagi et al., 2021; Zukin et al., 2021). For example, studies from the U.S. and China observed positive associations of first, second, or third trimester monoethyl phthalate (MEP) concentrations with excessive total GWG (Gao et al., 2021; James-Todd et al., 2016; Zukin et al., 2021) and first trimester GWG (Bellavia et al., 2017). However, other studies reported that elevated second trimester urinary levels of low molecular weight phthalate biomarkers (driven by MEP) are associated with inadequate total GWG (Philips et al., 2020). Additionally, a study from China reported lower first or second trimester MEP concentrations among women with inadequate compared to those with adequate total GWG (Li et al., 2019a).
It has become increasingly important to understand the cumulative impacts of phthalates/replacements and the role of each individual chemical within the context of the others. Of the few studies evaluating cumulative associations of phthalate biomarker mixtures with GWG, one study from China reported that a hazard index estimating exposures to di(2-ethylhexyl) phthalate (DEHP), diethyl phthalate (DEP), and di-n-butyl phthalate (DBP) across all pregnancy trimesters was associated with higher odds of excessive total GWG with most prominent associations observed for first trimester phthalate biomarkers (Gao et al., 2021). However, a Boston study observed no associations between sums of first, second, and third trimester phthalate metabolites of parents used in personal care products (MEP and monobutyl phthalate (MBP)), or phthalate metabolites shown to have anti-androgenic activity (MBP, monobenzyl phthalate (MBzP), mono-isobutyl phthalate (MiBP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2-ethylhexyl) phthalate (MEHP)) with total GWG (James-Todd et al., 2016). Finally, using Bayesian Kernel Machine Regression (BKMR), a study of U.S. women observed that a mixture of select first trimester phthalate, bisphenol, and paraben biomarker concentrations was positively associated with total GWG, and this association was primarily driven by the molar sum of four DEHP metabolites (Tyagi et al., 2021). BKMR is a method that can estimate the health effects of a complex mixture composed of highly correlated chemical exposures (Bobb et al., 2015).
Additional studies are needed to better understand the individual and cumulative associations of phthalate metabolite biomarkers with GWG. Furthermore, previous studies only evaluated a limited number of phthalates. Studies assessing a larger panel of phthalates to which pregnant women are exposed, as well as common plasticizer replacements (such as DiNCH and DEHTP), are needed. Therefore, our overall objective was to evaluate associations of phthalate/replacement biomarkers with GWG through late pregnancy by considering phthalate biomarkers individually and as a mixture. We hypothesized that individually and as a mixture, phthalates/replacements would be associated with higher GWG. GWG is a complex phenotype with both maternal and fetal contributions (Warrington et al., 2018), and fetal sex appears to be an important determinant of GWG (Caulfield et al., 1996; Grunebaum et al., 2014; Mando et al., 2016; Springel et al., 2016). Therefore, our secondary objective was to determine if these associations differed by fetal sex. We hypothesized that individually and as a mixture, associations of phthalates/replacements would differ by fetal sex.
2. MATERIALS AND METHODS
2.1. Illinois Kids Development Study (I-KIDS) recruitment and enrollment
The current study includes pregnant women recruited from two local obstetric clinics in Champaign-Urbana, Illinois who were invited to participate in I-KIDS – a prospective pregnancy and birth cohort with the overarching aim of evaluating the impacts of prenatal chemical exposures on infant neurodevelopment. Recruitment and enrollment have been described in detail elsewhere (Pacyga et al., 2021). I-KIDS includes women who were ≤ 15 weeks pregnant at enrollment, 18-40 years old, fluent in English, in a low-risk, singleton pregnancy, living within a 30-minute drive of the University of Illinois campus, and not planning to move out of the area before their child’s first birthday. The current study includes a sample of 303 women who enrolled in I-KIDS between February 2015 and August 2018, remained in the study through the birth of their infant, and have measurable concentrations of at least one urinary phthalate or replacement metabolite and pre- and late-pregnancy weights to calculate GWG through late pregnancy. As we have previously described, women in the current analytic sample are representative of the full I-KIDS cohort (Pacyga et al., 2022). All women provided written informed consent, and the study was approved by the Institutional Review Board at the University of Illinois. The analysis of de-identified specimens at the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects’ research.
2.2. Assessment of urinary phthalate/replacement biomarker concentrations
Women collected at least three and up to five first-morning urine samples in polypropylene urine cups at 8-15, 13-22, 19-28, 25-33, 32-40 weeks gestation (median 13, 17, 23, 28, and 34 weeks gestation, respectively), which corresponded with study home visits or routine prenatal care visits. Most of the 303 women contributed all five urine samples (94%), whereas 6% contributed three or four urine samples. Specifically, 99% of women provided a urine sample at 8-15 weeks gestation, 100% at 13-22 weeks gestation, 98% at 19-28 weeks gestation, 98% at 25-33 weeks gestation, and 98% at 32-40 weeks gestation. We have previously described urine collection, processing, and storage protocols in detail (Pacyga et al., 2021; Pacyga et al., 2022). For the current study, we quantified phthalate/replacement metabolite biomarker concentrations and measured specific gravity from pooled samples of up to five individual first-morning urines collected from each woman. Pooled samples were shipped overnight to the CDC Division of Laboratory Sciences in two batches in chronological order of participant enrollment as follows: enrolled February 2015 - July 2016 and enrolled July 2016 - August 2018. Using previously published methods with rigorous quality control/quality assurance protocols and excellent long-term reproducibility (Silva et al., 2013; Silva et al., 2007; Silva et al., 2019), the following 19 phthalate/replacement metabolites were quantified: monocarboxynonyl phthalate (MCNP), monocarboxyoctyl phthalate (MCOP), monooxononyl phthalate (MONP), monoisononyl phthalate (MiNP), MEHP, MEHHP, MEOHP, MECPP, mono-(3-carboxypropyl) phthalate (MCPP), MBzP, MBP, mono-hydroxybutyl phthalate (MHBP), MiBP, mono-hydroxy-isobutyl phthalate (MHiBP), MEP, cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester (MCOCH), cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester (MHiNCH), mono(2-ethyl-5-hydroxyhexyl) terephthalate (MEHHTP), and mono(2-ethyl-5-carboxypentyl) terephthalate (MECPTP).
2.3. Collection and calculation of GWG z-scores through late pregnancy
Within 24 hours of delivery, women reported their measured weight (in pounds) and the date of their most recent obstetric appointment (median: 38, range: 28 – 41 weeks gestation). We subtracted pre-pregnancy weight (which was self-reported in a home interview at 8 – 15 weeks gestation) from the weight reported at the last obstetric appointment before delivery to calculate GWG through late pregnancy (in kg). In the few cases where weight at the last obstetric visit prior to delivery was not available (n = 17), we used the reported weight from an earlier obstetric visit (median: 34, range: 29 – 37 weeks gestation). We calculated pre-pregnancy BMI- and gestational age-specific GWG z-scores based on previously validated methods using a reference population of pregnant women from Europe, North America, and Oceania (Santos et al., 2018).
2.4. Collection of maternal sociodemographic, lifestyle, and health information
After enrollment (8 – 15 weeks gestation), study staff conducted home visits to interview women about their race/ethnicity, educational attainment, annual household income, parity, smoking in the first trimester, alcohol intake in the first trimester, as well as pre-pregnancy weight and height. Self-reported pre-pregnancy weight and height were used to calculate pre-pregnancy BMI (kg/m2). To ascertain early pregnancy stress status, women completed the Perceived Stress Scale, a 10-item questionnaire that asks about thoughts and feelings during the past month (Cohen et al., 1983; Cohen and Williamson, 1988). At 8 – 15 and 32 – 40 weeks gestation, women also completed a semi-quantitative food frequency questionnaire (FFQ) that was adapted from the full length Block-98 FFQ (NutritionQuest, Berkeley, CA) and validated in a pregnant population (Bodnar and Siega-Riz, 2002; Boucher et al., 2006; Laraia et al., 2007). Reported dietary intakes representing diet during the previous three months were used to calculate the mean of early and mid-to-late pregnancy Alternative Healthy Eating Index (AHEI-2010). The AHEI-2010 is an 11-component diet quality index (out of 110 total points) based on foods and nutrients shown to be predictive of chronic disease risk and mortality, where a higher score indicates better overall diet quality (Chiuve et al., 2012; McCullough et al., 2002).
2.5. Statistical analysis
Derivation of the analytic sample is presented in Supplemental Figure 1. Out of 303 women with data on concentrations of all phthalate/replacement biomarkers and GWG z-scores, 299 (148 and 151 carrying males and females, respectively) were included in final covariate-adjusted single- and multi-pollutant analyses. Characteristics of the sample are presented as n (%) or median (range).
We evaluated specific gravity-adjusted phthalate/replacement biomarkers as molar sums or individual metabolites, which reflect exposures to 10 phthalate/replacement parent compounds (Pacyga et al., 2021; Pacyga et al., 2022). We created phthalate/replacement molar sums (in nmol/mL) by summing metabolites from common precursors as follows: MEHP, MEHHP, MEOHP, and MECPP for the sum of DEHP metabolites (ƩDEHP); MCOP, MiNP, and MONP for the sum of metabolites of di-isononyl phthalate (ƩDiNP); MBP and MHBP for the sum of DBP metabolites (ƩDBP); MiBP and MHiBP for the sum of di-iso-butyl phthalate metabolites (ƩDiBP); MHiNCH and MCOCH for the sum of DiNCH metabolites (ƩDiNCH); and sum of DEHTP metabolites MEHHTP and MECPTP to create ƩDEHTP. Specifics about equations are published elsewhere (Pacyga et al., 2021). Molar concentrations were back-converted to ng/mL by multiplying ƩDEHP, ƩDiNP, ƩDBP, ƩDiBP, ƩDiNCH, and ƩDEHTP by the molecular weights of MECPP, MCOP, MBP, MiBP, MHiNCH, and MECPTP, respectively (Pacyga et al., 2022; Rodriguez-Carmona et al., 2020; Zhang et al., 2020). We estimated exposure to di-isodecyl phthalate, di-n-octyl phthalate, benzylbutyl phthalate (BBzP), and DEP using ng/mL concentrations of their corresponding urinary metabolites MCNP, MCPP, MBzP, and MEP, respectively.
Based on the previous literature, we considered an extensive number of potential covariates in statistical models evaluating associations of phthalate/replacement biomarkers with GWG z-scores (Bellavia et al., 2017; Gao et al., 2021; James-Todd et al., 2016; Li et al., 2019a; Philips et al., 2020; Tyagi et al., 2021; Zukin et al., 2021). We generated a directed acyclic graph (DAG, Supplemental Figure 2) (VanderWeele and Robins, 2010), which included covariates that we and others found to be associated with both phthalate/replacement biomarkers and GWG z-scores. We used the DAG to guide the minimum sufficient adjustment set of covariates (VanderWeele and Robins, 2010). We assessed correlations between covariates to test for potential multicollinearity, but all covariates were only weakly or moderately correlated (r < 0.4; data not shown). Therefore, all final covariate-adjusted single-pollutant and mixtures models accounted for race/ethnicity, educational attainment, annual household income, smoking in the first trimester, pre-pregnancy BMI, and maternal diet quality. Annual household income, pre-pregnancy BMI, and maternal diet quality were included as continuous variables, whereas race/ethnicity, educational attainment, and smoking in the first trimester were categorized with the reference groups indicated in Table 1. We specified models that additionally accounted for perceived stress, parity, and alcohol intake in the first trimester, but our results were relatively unchanged with the addition of these variables (data not shown).
Table 1.
Characteristics of the study sample (n=303).
| Category | n (%) or median (min - max) |
|---|---|
| Age, years | 31.0 (18.0 - 40.0) |
| 1 Race/ethnicity | |
| Non-Hispanic white (ref) | 247 (81.5) |
| 2Others | 56 (18.5) |
| 1 Educational attainment | |
| Some college or less | 52 (17.2) |
| College grad or higher (ref) | 251 (82.8) |
| 1 Annual household income | |
| < $60,000 | 82 (27.2) |
| $60,000 - $99,999 | 113 (37.5) |
| ≥ $100,000 | 106 (35.2) |
| Parity | |
| 0 children | 161 (53.2) |
| 1 child | 94 (31.0) |
| ≥ 2 children | 48 (15.8) |
| 1 Smoking in the first trimester | |
| No (ref) | 288 (95.1) |
| Yes | 15 (4.9) |
| Alcohol intake in the first trimester | |
| None | 176 (58.1) |
| Any alcohol consumed | 127 (41.9) |
| 1 Pre-pregnancy BMI | |
| Under-/normal weight (< 25 kg/m2) | 158 (52.2) |
| Overweight (25 - 29.9 kg/m2) | 78 (25.7) |
| Obese (≥ 30 kg/m2) | 67 (22.1) |
| Perceived stress in the first trimester | |
| Low stress (0-13 pts) | 188 (62.7) |
| Moderate stress (14-26 pts) | 106 (35.3) |
| High stress (27-40 pts) | 6 (2.0) |
| Fetal sex | |
| Female | 153 (50.5) |
| Male | 150 (49.5) |
| Birth weight (grams) | |
| Females | 3360.8 (2353.0, 4507.6) |
| Males | 3611.7 (2588.3, 4394.2) |
| AHEI-2010 | 55.9 (28.1 - 80.2) |
| GWG through late pregnancy (kg) | 15.0 (−10.9 - 38.1) |
| GWG through late pregnancy (z-scores) | 0.5 (−3.0 - 4.1) |
| Gestational age at pre-delivery weight report (weeks) | 38.9 (28.9 - 41.9) |
Included in final statistical models as covariates.
Includes Hispanic, Black, Asian, American Indian, Multiracial, Other. Percentages may not add up to 100% due to missing. Subset sample (n missing): annual household income, GWG z-scores, gestational age at late pregnancy weight (2 missing); perceived stress (3 missing). AHEI-2010, Alternative Healthy Eating Index 2010 scored out of 110; GWG, gestational weight gain.
2.5.1. Primary and sensitivity single-pollutant analyses
To address our main objectives, we first specified multivariable linear regression models to evaluate single-pollutant associations of phthalate/replacement biomarkers with GWG z-scores. All phthalate/replacement biomarkers were ln-transformed because of their right-skewed distributions. For concentrations below the limit of detection (LOD) we used instrumental reading values to avoid bias associated with imputing values <LOD (Succop et al., 2004). Across the individual and molar sum biomarkers described above, only one woman had a concentration of zero for the two DiNCH metabolites MCOCH and MHiNCH. Therefore, we used the following equation [ln(ƩDiNCH + 1)] to avoid undefined estimates for ƩDiNCH. We did not transform GWG z-score because it was normally distributed. Regression diagnostics based on residuals were conducted to ensure all model assumptions were met. Because women with GWG below zero (n=4) could have unique characteristics that influence our results, we also conducted sensitivity analyses, using linear regression models, that excluded these women. For all linear regression analyses, the resulting β-estimates and 95% confidence intervals (CIs) represent the change in GWG z-scores for every interquartile range (IQR) increase in phthalate/replacement biomarker concentration.
2.5.2. Primary mixture analyses
To evaluate associations of phthalate/replacement biomarkers as a joint mixture with GWG z-scores, we first estimated quantile-based g-computation (QGComp) models (Keil et al., 2020). QGComp was adapted from weighted quantile sum regression (WQSR) (Carrico et al., 2015) and relaxes the assumption that all chemical exposures are associated with the outcome in the same direction. For our analyses, we included the following 10 individual or molar sum phthalate/replacement biomarkers in our mixture index: MCNP, ƩDiNP, ƩDEHP, MCPP, MBzP, ƩDBP, ƩDiBP, MEP, ƩDiNCH, and ƩDEHTP – these 10 components represent common phthalates or phthalate replacement compounds to which I-KIDS women are exposed. Then, QGComp was used to estimate the joint association of the mixture index with GWG z-scores (not transformed) via multiple linear regression, and determine the relative weights for each phthalate/replacement biomarker that indicate the contribution of each biomarker to the joint association. We generated results without bootstrapping to obtain the partial negative associations (i.e., scaled sum of negative biomarker weights) and partial positive associations (i.e., scaled sum of positive biomarker weights). As we transformed all phthalate/replacement biomarker concentrations into deciles, the resulting β-estimates and 95% CIs are interpreted as the change in GWG z-scores if concentrations of all phthalate/replacement biomarkers simultaneously changed by 10%. However, to make these results comparable to those from single-pollutant models, we multiplied the β-estimates and 95% CIs by 5.0 to represent the change in GWG z-scores for each IQR change in the mixture. The most prominent contributors to mixture associations were determined by the strength of the relative weights.
One limitation of QGComp is that it assumes that all chemicals included in the mixture originate from a single source of exposure and, hence, it does not allow exposures to vary independently. For this reason, we chose to additionally evaluate associations of phthalates/replacement biomarkers as a mixture with GWG z-scores using WQSR, as these chemicals have distinct sources and may have different mechanisms of action. We specified WQSR models with the repeated holdout approach, which improves the stability of results by combining cross-validation and bootstrap resampling to implement WQSR in an iterative fashion (Carrico et al., 2015; Tanner et al., 2019). Briefly, WQSR is a supervised mixture method that creates a unidirectional mixture index by transforming exposures into quantiles, and then evaluates the cumulative association of the mixture index with the outcome via multiple linear regression (Carrico et al., 2015; Czarnota et al., 2015). We included the same 10 phthalate/replacement biomarkers in the mixture index and converted concentrations into deciles. GWG z-scores were not transformed. We generated a distribution of results using 100 iterations (repeated holdouts), each with 100 bootstrap replications. Within each of the 100 iterations, data were randomly split 40/60% into training and validation datasets, respectively. WQSR weighs the relative variable importance of each individual mixture component, which can be used to determine the largest contributors to the cumulative association (Carrico et al., 2015; Czarnota et al., 2015). Biomarkers with mean weights above 0.10 (1/10 biomarkers included in the mixture) were identified as the largest contributors. Because WQSR models are specified to evaluate associations between all mixture components and the outcome in one direction at a time, the models assume all components are associated with the outcome in the same direction, and all component weights are non-negative and sum to one. Therefore, we explored WQSR models separately in the negative and positive directions.
Then, using findings from single-pollutant and total WQSR models, we determined which phthalate/replacement biomarkers tended to be positively and negatively associated with GWG z-scores. WQSR assumes that all biomarkers in the mixture are associated with the outcome in the same direction – a rather strong assumption not supported by our findings. Therefore, after identifying the biomarkers that were positively associated with GWG z-scores, we multiplied their concentrations by (−1.0), thus reversing the order of their quantiles. Next, all 10 phthalate/replacement biomarkers were included in our mixture index using the updated scoring scheme, and we evaluated WQSR models in the negative direction using the same settings as described previously. From the 100 repeated holdout samples, we obtained the mean cumulative association of phthalate/replacement biomarker concentrations with GWG z-scores, as well as weights for each biomarker in the mixture that indicate its relative importance as a mixture component. The resulting β-estimate and 95% CIs (which we also multiplied by 5.0) can be interpreted as the change in GWG z-scores (not-transformed) if concentrations of all negative biomarkers increased by one IQR and those of all positive biomarker concentrations simultaneously decreased by one IQR. Phthalate/replacement biomarker weights were renormalized, so that the weights of all the positive biomarkers (i.e., partial positive association) and the weights all the negative biomarkers (i.e. partial negative association) separately summed to 1.0.
2.5.3. Secondary analyses
We performed several secondary analyses to evaluate fetal sex-specific associations. First, we conducted linear regression analyses to evaluate single-pollutant associations of ln-transformed phthalate/replacement biomarker concentrations with GWG z-scores stratified by fetal sex. GWG z-scores were operationalized as discussed in section 2.5.1. To obtain fetal sex-specific β-estimates and 95% CIs, we included a multiplicative interaction between the phthalate/replacement biomarker and fetal sex. We reported all results regardless of the significance of the interaction P-value (Pbiomarker*sex) and compared the direction, strength, and precision of associations to identify meaningful differences by fetal sex. Second, we evaluated fetal sex-specific associations of the phthalate/replacement biomarker mixture with GWG z-scores by specifying separate QGComp and WQSR models for women carrying females and males as we described previously.
When evaluating associations between risk factors and GWG, it is recommended that studies include pre-pregnancy BMI as an effect modifier (Hutcheon and Bodnar, 2018). Therefore, as an additional sensitivity analysis, we first evaluated single-pollutant associations of phthalate/replacement biomarkers with GWG z-scores by pre-pregnancy BMI. To obtain the pre-pregnancy BMI-specific β-estimates and 95% CIs in linear regression analyses, we included a multiplicative interaction between the phthalate/replacement biomarker and pre-pregnancy BMI. Second, because we hypothesized that associations between phthalate/replacement biomarkers with GWG z-scores are fetal sex-specific, we also conducted linear regression analyses including a multiplicative three-way interaction (and all relevant two-way interactions) between the phthalate/replacement biomarker, pre-pregnancy BMI, and fetal sex. As discussed earlier, we reported all results regardless of the significance of the interaction P-value (Pint).
We performed linear regression analyses in SAS version 9.4 (SAS Institute Inc, Cary, NC) using PROC GLM. We performed QGComp and WQSR analyses in R Statistical Software (v4.1.1; R Core Team 2021) using R packages “qgcomp: Quantile G-Computation” (Keil, 2022) and “gWQS: Generalized Weighted Quantile Sum Regression” (Ranzetti, 2021) respectively. Rather than focusing on statistical significance, we focused on patterns (direction and magnitude) and precision of observed associations based on recommendations from the American Statistical Association and others (Amrhein et al., 2019; Wasserstein and Lazar, 2016). Thus, in addition to results where the upper and lower limits do not cross the null (zero), we also considered results as potentially meaningful if either the upper or lower confidence limit crossed, but were close to the null, and if the confidence interval was narrow relative to other estimates. For our mixtures findings, our overall conclusions for notable phthalate/replacement biomarker contributors were based on identifying consistent findings from both QGComp and WQSR, at least in the qualitative sense. Additionally, β-estimates and 95% CIs for a z-score difference in GWG can be converted (considering the gestational age at late pregnancy weight and pre-pregnancy BMI) to represent a difference in kg (Hutcheon and Bodnar, 2018). As a reference, a 0.2 z-score difference in weight gain through 38 weeks gestation can be interpreted approximately as a 0.82 kg difference for underweight, 0.84 difference for normal weight, 1.09 kg difference for overweight, 1.18 kg difference for obese class I, 1.28 kg difference for obese class II, and 1.44 kg difference for obese class III pre-pregnancy BMI.
3. RESULTS
3.1. Characteristics of the I-KIDS population
Women had a median age of 31 years (Table 1). Most women were non-Hispanic white (82%), college-educated (83%), nulliparous (53%), and had annual household incomes > $60,000 (73%). Most women did not smoke (95%) or consume alcohol (58%) in the first trimester, around half had overweight or obesity before pregnancy (48%), and median AHEI-2010 was 55.9 (range: 28.1 – 80.2). Around two thirds of women reported having low perceived stress, whereas the rest reported having moderate or high perceived stress in the first trimester. Median GWG through median 38 weeks gestation was 15.0 kg (range: −10.9 – 38.1).
3.2. Urinary phthalate/replacement metabolite biomarker concentrations in I-KIDS
The distribution of urinary phthalate/replacement biomarker concentrations are presented in Table 2. Greater than 96% of women had detectable concentrations of at least one phthalate metabolite per parent compound (including DEHTP), and > 90% of women had detectable concentrations of at least one DiNCH metabolite biomarker. As we have shown previously, most phthalate/replacement biomarkers were weakly-to-moderately correlated with each other (r < 0.4), though we did observe strong correlations between ƩDiNP and MCPP (r > 0.8) (Pacyga et al., 2022). Additionally, as reported previously, median uncorrected urinary phthalate/DiNCH biomarker concentrations in I-KIDS were similar to those of same age women from the National Health and Nutrition Examination Survey (NHANES) during a similar time period (Pacyga et al., 2022). However, DEHTP metabolite concentrations in I-KIDS were 1.5 to 3 times higher than those from NHANES (Pacyga et al., 2022).
Table 2.
Distribution of maternal urinary specific gravity-adjusted phthalate/replacement biomarker concentrations (n = 303).
| Parent | Biomarker | % detectable |
25th percentile (ng/mL) |
50th percentile (ng/mL) |
75th percentile (ng/mL) |
|---|---|---|---|---|---|
| Di-isodecyl phthalate, DiDP | MCNP * | 100.0 | 1.33 | 1.81 | 2.58 |
| Di-isononyl phthalate, DiNP | ƩDiNP * | -- | 4.94 | 7.96 | 15.11 |
| MCOP | 100.0 | 4.47 | 7.09 | 13.65 | |
| MiNP | 31.4 | 0.4 | 0.62 | 1.09 | |
| MONP | 100.0 | 1.71 | 2.64 | 4.63 | |
| Di(2-ethylhexyl) phthalate, DEHP | ƩDEHP * | -- | 14.75 | 19.71 | 29.89 |
| MEHP | 75.6 | 0.85 | 1.32 | 2.13 | |
| MEHHP | 100.0 | 3.62 | 5.40 | 8.32 | |
| MEOHP | 100.0 | 2.88 | 4.00 | 6.34 | |
| MECPP | 100.0 | 6.08 | 8.32 | 12.64 | |
| Di-n-octyl phthalate, DOP | MCPP * | 96.0 | 0.86 | 1.30 | 1.90 |
| Benzylbutyl phthalate, BBzP | MBzP * | 99.3 | 2.54 | 5.16 | 10.00 |
| Di-n-butyl phthalate, DBP | ƩDBP * | -- | 9.36 | 14.05 | 19.22 |
| MBP | 100.0 | 8.46 | 12.68 | 17.41 | |
| MHBP | 90.1 | 0.75 | 1.22 | 1.87 | |
| Di-iso-butyl phthalate, DiBP | ƩDiBP * | -- | 7.86 | 11.56 | 19.59 |
| MiBP | 99.7 | 5.74 | 8.53 | 14.40 | |
| MHiBP | 99.7 | 2.13 | 3.11 | 5.47 | |
| Diethyl phthalate, DEP | MEP * | 100.0 | 14.12 | 27.47 | 47.79 |
| Diisononyl-cyclohexane-1,2-dicarboxylate, DiNCH | ƩDiNCH * | -- | 1.16 | 1.88 | 3.43 |
| MHiNCH | 90.7 | 0.69 | 1.14 | 2.26 | |
| MCOCH | 67.3 | 0.48 | 0.71 | 1.22 | |
| Di(2-ethylhexyl) terephthalate, DEHTP | ƩDEHTP * | -- | 30.18 | 69.65 | 158.54 |
| MEHHTP | 100.0 | 3.84 | 8.16 | 20.11 | |
| MECPTP | 100.0 | 25.69 | 58.88 | 135.53 |
All concentrations are presented in ng/mL. All metabolite concentrations were specific gravity-adjusted. Molar sums were converted back to ng/mL by multiplying each molar sum by the molecular weight of a corresponding major metabolite as discussed in the statistical analysis section.
Indicates the biomarkers of interest for the current study. MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MCNP, monocarboxynonyl phthalate; MCOCH, cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester; MCOP, monocarboxyoctyl phthalate; MCPP, mono-(3-carboxypropyl) phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MECPTP, mono(2-ethyl-5-carboxypentyl) terephthalate; MEHP, mono(2-ethylhexyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEHHTP, mono(2-ethyl-5-hydroxyhexyl) terephthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate; MHBP, mono-hydroxybutyl phthalate; MHiBP, mono-hydroxy-isobutyl phthalate; MHiNCH, cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester; MiBP, mono-isobutyl phthalate; MiNP, monoisononyl phthalate; MONP, monooxononyl phthalate; ƩDiNP, sum of di(isononyl) phthalate metabolites; ƩDEHP, sum of di(2-ethylhexyl) phthalate metabolites; ƩDBP, sum of di-n-butyl phthalate metabolites; ƩDiBP, sum of di-iso-butyl phthalate metabolites; ƩDiNCH, sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites; ƩDEHTP, sum of di(2-ethylhexyl) terephthalate metabolites.
3.3. Associations of individual phthalate/replacement biomarkers with GWG z-scores
In single-pollutant linear regression models, ƩDEHP and ƩDEHTP were inversely associated with GWG z-scores (Table 3). For example, IQR increases in ƩDEHP and ƩDEHTP were associated with −0.16 (95% CI: −0.29, −0.03) and −0.20 (95% CI: −0.38, −0.02) lower GWG z-scores, respectively. MCPP and ƩDiNCH were also meaningfully associated with GWG z-scores, where IQR increases in MCPP and ƩDiNCH were associated with −0.09 (95% CI: −0.21, 0.03) and −0.11 (95% CI: −0.22, 0.01) lower GWG z-scores, respectively. These results persisted in sensitivity analyses excluding the four women with GWG < 0 (Supplemental Table 1). We also observed suggestive differences in associations between some phthalate/replacement biomarkers and GWG z-scores by pre-pregnancy BMI (Supplemental Table 2). However, when additionally evaluating differences by pre-pregnancy BMI and fetal sex, no definitive patterns emerged and results were notably less precise (Supplemental Table 3).
Table 3.
Single-pollutant associations of phthalate/replacement biomarkers with GWG z-scores.
| All women (n = 299) |
Women carrying males (n = 148) |
Women carrying females (n = 151) |
||
|---|---|---|---|---|
| Biomarker | β (95% CI) | β (95% CI) | β (95% CI) | P biomarker*sex |
| MCNP | 0.00 (−0.12, 0.13) | 0.03 (−0.16, 0.23) | 0.00 (−0.16, 0.17) | 0.80 |
| ƩDiNP | 0.01 (−0.13, 0.15) | 0.25 (0.02, 0.49) | −0.08 (−0.25, 0.09) | 0.02 |
| ƩDEHP | −0.16 (−0.29, −0.03) | 0.04 (−0.14, 0.22) | −0.35 (−0.53, −0.18) | 0.002 |
| MCPP | −0.09 (−0.21, 0.03) | −0.03 (−0.22, 0.17) | −0.11 (−0.27, 0.05) | 0.49 |
| MBzP | 0.09 (−0.06, 0.25) | 0.15 (−0.08, 0.37) | 0.03 (−0.18, 0.25) | 0.46 |
| ƩDBP | −0.09 (−0.22, 0.05) | −0.08 (−0.28, 0.11) | −0.10 (−0.27, 0.07) | 0.90 |
| ƩDiBP | −0.03 (−0.16, 0.10) | 0.01 (−0.20, 0.23) | −0.05 (−0.22, 0.12) | 0.64 |
| MEP | −0.08 (−0.24, 0.07) | 0.00 (−0.22, 0.22) | −0.13 (−0.35, 0.09) | 0.39 |
| ƩDiNCH | −0.11 (−0.22, 0.01) | −0.17 (−0.35, 0.01) | −0.04 (−0.19, 0.11) | 0.27 |
| ƩDEHTP | −0.20 (−0.38, −0.02) | −0.26 (−0.50, −0.02) | −0.12 (−0.37, 0.13) | 0.43 |
Data are presented as the change (95% CI) in GWG z-scores for every IQR increase in phthalate/replacement biomarker concentration. Models accounted for race/ethnicity, educational attainment, annual household income, smoking in the 1st trimester, pre-pregnancy BMI, and maternal diet quality. To obtain fetal sex-specific estimates and the interaction P-value (Pbiomarker*sex), we additionally included a multiplicative interaction between phthalate/replacement biomarker and fetal sex. Cl, confidence interval; GWG, gestational weight gain; MCNP, monocarboxynonyl phthalate; ƩDiNP, sum of di(isononyl) phthalate metabolites; ƩDEHP, sum of di(2-ethylhexyl) phthalate metabolites; MCPP, mono-(3-carboxypropyl) phthalate; MBzP, monobenzyl phthalate; ƩDBP, sum of di-n-butyl phthalate metabolites; ƩDiBP, sum of di-iso-butyl phthalate metabolites; MEP, monoethyl phthalate; ƩDiNCH, sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites; ƩDEHTP, sum of di(2-ethylhexyl) terephthalate metabolites.
3.4. Associations of phthalate/replacement biomarker mixture with GWG z-scores
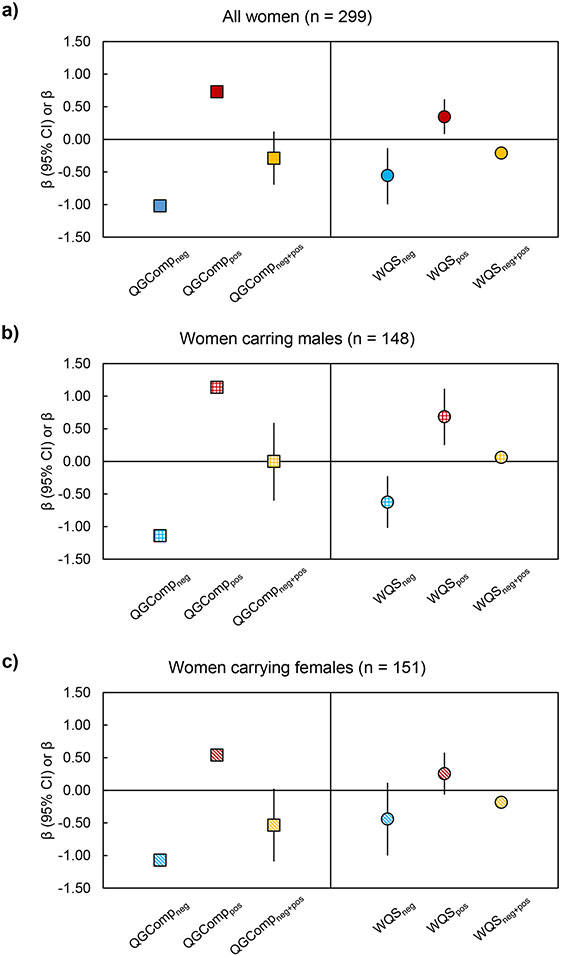
When using QGComp to evaluate the joint association of the phthalate/replacement biomarker mixture with GWG z-scores, we observed that simultaneous IQR increases in all biomarker concentrations in the mixture were marginally associated with lower GWG z-scores (β: −0.29; 95% CI: −0.70, 0.12) (Figure 1a and Supplemental Table 4), as the partial negative (β: −1.02) and positive (β: 0.73) associations were similar in strength. The largest phthalate/replacement biomarker contributors to partial negative associations were ƩDEHP (28%), ƩDEHTP (23%), and MCPP (18%), whereas the largest biomarker contributors to partial positive associations were ƩDiNP (64%) and MBzP (36%) (Table 4).
Fig. 1.
Joint associations of phthalate/replacement biomarkers with GWG z-scores in a) all women, b) women carrying males, and c) women carrying females. QGComp models and WQSR models using partial reverse scoring approach accounted for race/ethnicity, educational attainment, annual household income, smoking in the 1st trimester, pre-pregnancy BMI, and maternal diet quality. Data are presented as the change (square for QGComp, circle for WQSR models) and 95% CI (vertical black lines) in GWG z-scores for an IQR change in the mixture. Separate models were specified for all women (n = 299), women carrying males (n = 148), and women carrying females (n = 151). QGCompneg, scaled effect size in the negative direction; QGComppos, scaled effect size in the positive direction; QGCompneg+pos, joint association as the sum of QGCompneg and QGComppos. WQSneg and WQSpos, sum of negative and positive weights, respectively, calculated after renormalizing positive and negative weights to 1.0; WQSneg+pos, joint association as the sum of WQSneg and WQSpos. GWG, gestational weight gain; QGComp, quantile g-computation; WQSR, weighted quantile sum regression.
Table 4.
Relative weights from reverse scoring WQSR and QGComp models evaluating associations of the phthalate/replacement mixture with GWG z-scores.
| All women (n = 299) |
Women carrying males (n = 148) |
Women carrying females (n = 151) |
||||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | |
| Method | Chemical (wt) | Chemical (wt) | Chemical (wt) | Chemical (wt) | Chemical (wt) | Chemical (wt) |
| QGComp 1 |
ƩDEHP (0.284) ƩDEHTP (0.233) MCPP (0.175) MCNP (0.117) MEP (0.094) ƩDiNCH (0.046) ƩDiBP (0.032) ƩDBP (0.020) |
ƩDiNP (0.639)
MBzP (0.361) |
ƩDBP (0.304) ƩDEHTP (0.292) MCPP (0.151) MCNP (0.097) ƩDiNCH (0.087) MEP (0.036) ƩDEHP (0.034) |
ƩDiNP (0.518) MBzP (0.386) ƩDiBP (0.096) |
ƩDEHP (0.553) ƩDEHTP (0.122) MEP (0.118) MCNP (0.076) ƩDiBP (0.072) ƩDiNCH (0.038) MCPP (0.022) |
ƩDiNP (0.372) MBzP (0.355) ƩDBP (0.273) |
| WQSR Reverse Scoring Method 2 |
ƩDEHP (0.250) ƩDEHTP (0.190) MCPP (0.175) MEP (0.128) ƩDiNCH (0.113) ƩDiBP (0.075) ƩDBP (0.070) |
ƩDiNP (0.500) MBzP (0.427) MCNP (0.073) |
ƩDBP (0.383) ƩDEHTP (0.255) MCPP (0.203) ƩDiNCH (0.159) |
ƩDiNP (0.358) MBzP (0.249) ƩDiBP (0.136) MCNP (0.094) MEP (0.092) ƩDEHP (0.073) |
ƩDEHP (0.372) MEP (0.177) ƩDiBP (0.153) ƩDiNCH (0.139) MCPP (0.084) ƩDiNP (0.074) |
ƩDBP (0.342) MBzP (0.265) ƩDEHTP (0.199) MCNP (0.194) |
Separate models were specified for all women (n = 299), women carrying males (n=148), and women carrying females (n=151). Bolded chemicals are those that crossed the threshold (1/10 chemicals in the mixture).
Data are presented as the chemical (relative weight) from QGComp models.
Data are presented as the chemical (relative weight) from negatively constrained WQSR models using the reverse scoring method where chemicals that were positively associated with GWG z-scores (see positive column) were reverse coded; weights were renormalized to 1.0 within positive and negative columns. GWG, gestational weight gain; MCNP, monocarboxynonyl phthalate; ƩDiNP, sum of di(isononyl) phthalate metabolites; ƩDEHP, sum of di(2-ethylhexyl) phthalate metabolites; MCPP, mono-(3-carboxypropyl) phthalate; MBzP, monobenzyl phthalate; ƩDBP, sum of di-n-butyl phthalate metabolites; ƩDiBP, sum of di-iso-butyl phthalate metabolites; MEP, monoethyl phthalate; ƩDiNCH, sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites; ƩDEHTP, sum of di(2-ethylhexyl) terephthalate metabolites; QGComp, quantile g-computation; WQSR, weighted quantile sum regression; Wt, weight.
Results evaluating associations of all 10 phthalate/replacements as a cumulative mixture with GWG z-scores in all women using standard WQSR models are presented in Supplemental Tables 4 and 5. When evaluating mixture effects via WQSR using the (partial) reverse scoring approach, so that all biomarkers are associated with the outcome in the same direction, we observed that the full mixture was associated with GWG z-scores (β: −0.90; 95% CI: −1.60, −0.20). Decomposing this overall association into contributions from originally negative and positive mixture components, we observed that IQR increases in the negative phthalate/replacement biomarkers were associated with 0.56 (95% CI: 0.14, 1.00) reductions in GWG z-scores (Figure 1a), with the largest contributors being ƩDEHP (25%), ƩDEHTP (19%), and MCPP (18%) (Table 4). Correspondingly, IQR increases in positive biomarkers were associated with 0.35 (95% CI: 0.08, 0.62) increases in GWG z-scores (Figure 1a), with the largest contributors being ƩDiNP (50%) and MBzP (43%) (Table 4).
3.5. Fetal sex-specific associations of phthalate/replacement biomarkers with GWG z-scores
When evaluating associations using individual phthalate/replacement biomarkers, the inverse associations of ƩDiNCH and ƩDEHTP with GWG z-scores observed in all women appeared to be more robust in women carrying males, whereas inverse associations in women carrying females were modest (Table 3). However, inverse associations of ƩDEHP with GWG z-scores in all women were driven by women carrying females (β: −0.35, 95% CI: −0.53, −0.18), with no association observed in those carrying males (β: 0.04, 95% CI: −0.14, 0.22). Additionally, we identified a positive association between ƩDiNP and GWG z-scores in women carrying males (β: 0.25, 95% CI: 0.02, 0.49), with no association observed in those carrying females (β: −0.08, 95% CI: −0.25, 0.09).
Using QGComp, we observed that the phthalate/replacement biomarker mixture was marginally associated with lower GWG z-scores in women carrying females (β: −0.54; 95% CI; −1.09, 0.03) (Figure 1c), but not associated with GWG z-scores in women carrying males (β: 0.00; 95% CI: −0.60, 0.59) (Figure 1b). In women carrying females, ƩDEHP (55%) was identified as the largest biomarker contributor to the partial negative association (Table 4). However, in women carrying males, the magnitude of the partial negative (β: −1.14) and partial positive (β: 1.14) associations were equal in strength, resulting in no overall joint association of the mixture with GWG z-scores in this sub-sample (Figure 1b and Supplemental Table 4). In women carrying males, ƩDBP (30%) and ƩDEHTP (29%) were the largest contributors to partial negative associations, while ƩDiNP (52%) and MBzP (39%) were the largest contributors to partial positive associations (Table 4).
Results evaluating associations of all 10 phthalate/replacements as a cumulative mixture with GWG z-scores separately in women carrying males and females using standard WQSR models are presented in Supplemental Tables 4 and 5. When evaluating associations of the phthalate/replacement biomarker mixture with GWG z-scores using WQSR with the reverse scoring method, we observed that the biomarker mixture was associated with GWG z-scores both in women carrying males and females, but the association was much more robust in women carrying males (β: −1.30; 95% CI: −2.15, −0.50) than in those carrying females (β: −0.70; 95% CI: −1.55, 0.20) (Figure 1b,c and Supplemental Table 4). With regards to the partial negative associations stratified by fetal sex, we observed that IQR increases in the negative biomarkers were associated with GWG z-scores decreases of 0.63 (95% CI: 0.23, 1.02) and 0.44 (95% CI: −0.12, 1.00) in women carrying males and females, respectively. In women carrying males, the strongest phthalate/replacement biomarker contributors to associations in the negative direction were ƩDBP (39%) and ƩDEHTP (26%), whereas in women carrying females, the strongest contributors were ƩDEHP (37%) and MEP (18%) (Table 4). When evaluating partial positive associations by fetal sex, we observed that IQR increases in positive biomarkers were associated with 0.69 (95% CI: 0.25, 1.12) higher GWG z-scores in women carrying males (Figure 1b), with ƩDiNP (36%) and MBzP (25%) identified as the strongest contributors to this association (Table 4). In women carrying females, IQR increases in positive biomarkers were weakly associated with 0.26 (95% CI: −0.07, 0.58) higher GWG z-scores (Figure 1c), with ƩDBP (34%) was identified as the strongest contributor to this association (Table 4).
4. DISCUSSION
We observed that urinary biomarker concentrations of phthalate plasticizers and their replacements were associated with GWG z-scores, which was generally consistent between single-pollutant and mixture modeling approaches, as well as between QGComp and WQSR. In secondary single- and multi-pollutant analyses, we observed that some associations of phthalates/replacement biomarkers with GWG z-scores were fetal-sex-specific, a finding which has not been reported previously. While consistent marginal inverse associations were observed in women carrying females, in those carrying males, we observed equal associations in both the negative and positive directions. These results contribute to the growing evidence that exposure to phthalates may impact GWG in pregnant women and provide novel information about the potential for widely used plasticizer replacements, DiNCH and DEHTP, to impact GWG.
4.1. Individual phthalate/replacement biomarkers are associated with GWG z-scores
Contrary to our hypothesis, in our study, select phthalate biomarkers (ƩDEHP and MCPP) and biomarkers of replacements (ƩDiNCH and ƩDEHTP) were generally inversely associated with GWG z-scores, and for some of these biomarkers, associations were either more prominent in women carrying females (for ƩDEHP) or males (for ƩDiNCH and ƩDEHTP). Interestingly, a positive association emerged for ƩDiNP with GWG z-scores in women carrying males. It is important to view GWG as a complex phenotype, with contributions from the fetus, placenta, amniotic fluid, maternal fluid (i.e., blood, extracellular fluid), protein and fat storage, as well as uterine and breast tissues (Warrington et al., 2018). Our findings in women carrying females support the idea that some phthalates may target biological pathways that prevent weight gain in one or more of these storage depots (Rasmussen and Yaktine, 2009). In pregnancy, exposures to certain phthalates are associated with altered metabolic processes required for appropriate GWG, such as glucose and lipid homeostasis (Gaston et al., 2020; Muscogiuri et al., 2017). Specifically, in experimental models, phthalates have been shown to interact with peroxisome proliferator-activated receptor gamma, liver X receptors, and retinoid X receptors, which are important regulators of glucose and lipid homeostasis (Veiga-Lopez et al., 2018). These metabolic processes are regulated by sex-steroid hormones (such as estrogen and progesterone) and cytokines/adipokines produced by the maternal-fetal-placental unit (Rasmussen and Yaktine, 2009), which are also potential biological targets of phthalates/replacements (Gao et al., 2022; Pacyga et al., 2021; Qian et al., 2020; Strakovsky and Schantz, 2018a; Strakovsky and Schantz, 2018b; Wang et al., 2020; Welch et al., 2021). For example, phthalates have been shown to interfere with the regulation of the hypothalamic-pituitary-gonadal (HPG) axis by interacting with steroidogenic enzymes and by modulating the activity of hormone receptors (e.g. estrogen receptor, androgen receptor) (Hlisnikova et al., 2020). The hormone-mediated role of the placenta in GWG and the known sex differences in the placental response to phthalates may also explain our fetal sex-specific findings (Strakovsky and Schantz, 2018a; Strakovsky and Schantz, 2018b), although further studies are needed to elucidate the biological basis for these observations.
Nevertheless, appropriate GWG is critical for maternal health and fetal development (Rasmussen and Yaktine, 2009), and both insufficient and excessive GWG may have adverse implications for maternal and offspring health. For example, women with insufficient GWG may be more likely to experience postpartum depression (Zanardo et al., 2021), whereas those with excessive GWG are at higher risk of postpartum weight retention that could lead to cardiometabolic disease later in life (Haggerty et al., 2021). Babies of mothers with insufficient GWG have higher odds of being born pre-term and small-for-gestational age, whereas those of mothers with excessive total GWG have higher odds of macrosomia or being born large-for-gestational age (Goldstein et al., 2018). Importantly, these early birth phenotypes have been linked with the later development of respiratory problems, cognitive and/or behavioral deficits, and cardiometabolic disease in children (Gallacher et al., 2016; Hack et al., 1995; Palatianou et al., 2014; Torniainen et al., 2013). Additionally, GWG has also been shown to be associated with these same adverse childhood outcomes. Specifically, one study showed that compared to a GWG z-score of 0.0, a GWG z-score of +0.5 was associated with 2.2 (95% CI: 0.7, 3.7) excess cases of childhood overweight or obesity per 100 pregnancies (Bodnar et al., 2021). Another study found that compared to GWG z-scores between −1.0 and +1.0, children of women who had a GWG z-score over +1.0 spent 15.0 seconds (95% CI: 1.8, 28.0) longer completing a task measuring executive performance, suggesting that higher GWG is associated with poorer executive function performance in children (Pugh et al., 2015). Phthalates are also sex-specifically associated with many of these same short- and long-term infant and child health outcomes (Qian et al., 2020), so it is possible that associations of certain phthalates with GWG could partially explain relationships of phthalates with child and maternal health, and this should be explored further.
The current literature evaluating associations of individual phthalates with GWG is mixed, but also limited with regards to phthalate replacements and evaluating differences by fetal sex. Consistent with our findings, one study from the Netherlands evaluated GWG through late pregnancy and reported that associations of mid-pregnancy MCPP with GWG trended in the negative direction (Philips et al., 2020). However, most other studies evaluated total GWG using the IOM categories or trimester-specific GWG and quantified phthalate metabolite biomarkers from individual spot urines collected in early, mid, and/or late pregnancy, which limits direct comparisons to our study. Of the studies evaluating total GWG, two (from Anhui Province in China and Salinas Valley, California in the U.S.) observed that higher urinary metabolite concentrations of DEHP, DBP, and DEP in early/mid pregnancy were associated with higher total GWG and higher odds of excessive GWG (Gao et al., 2021; Zukin et al., 2021). Additionally, in pregnant women from Boston, mean urinary MEP concentrations were associated with higher odds of excessive total GWG, although this relation was non-monotonic (James-Todd et al., 2016). Conversely, in the previously discussed Netherlands cohort, higher early/mid gestation urinary low molecular weight phthalate biomarkers were associated with higher odds of insufficient total GWG (Philips et al., 2020), while in women from Hubei Province in China, MEP concentrations were lower among women with inadequate compared to adequate total GWG (Li et al., 2019b). Inconsistent findings among observational studies could also relate to covariates accounted for in statistical models and study population characteristics. For example, both our study and the Netherlands study adjusted for maternal diet, but this was not accounted for by other studies. Additionally, the majority of our women are non-Hispanic white, of higher socioeconomic status (SES), and almost half had overweight or obesity before pregnancy, which may also explain differences in findings from cohorts in China (where the majority of women were normal weight before pregnancy) and California (where most women were migrants from Mexico with lower SES). The experimental evidence related to maternal body weight gain is also mixed. For example, studies evaluating the effects of prenatal DEP exposure observed decreasing, increasing, and no effects on maternal body weight in response to DEP (Weaver et al., 2020). Another study observed that compared to controls, F0 generation dams exposed to DEHP (pre-conception to weaning), DiNP (mating to weaning, pre-conception to weaning), or DBP (pre-conception to weaning) gained more weight (Neier et al., 2019). Further research can assist in identifying what may be contributing to these conflicting findings within and between observational and experimental studies.
4.2. Phthalate/replacement biomarkers as a cumulative mixture are associated with GWG z-scores
Given that pregnant women are likely exposed to numerous phthalates/replacements, evaluating these chemicals as a cumulative mixture is important for understanding the aggregate association of many phthalates/replacement with pregnancy and fetal health (Braun et al., 2016). For the current study, we compared results from two widely used statistical mixtures methods, QGComp and WQSR (Czarnota et al., 2015; Keil et al., 2020). To make our findings more comparable to those from QGComp, while simultaneously satisfying the WQSR assumption that all mixture components are associated with the outcome in the same direction, we utilized a (partial) reverse scoring approach for WQSR. Although the two approaches have distinct purposes and properties, we generally observed very consistent findings across the two methods – most notably, there was almost perfect agreement regarding the ordering and direction of the contribution of key phthalate/replacement biomarkers to joint mixture associations. Specifically, in all women, we observed that the partial negative and positive associations were similar in magnitude, with the overall biomarker mixture only marginally inversely associated with GWG z-scores through late pregnancy. ƩDEHP, ƩDEHTP, and MCPP were identified as the largest contributors to inverse associations and ƩDiNP and MBzP identified as the most prominent contributors to positive associations. When stratifying by fetal sex, we observed marginal inverse associations in women carrying females, a result that was largely driven by ƩDEHP. However, in women carrying males, we observed equal partial positive (driven by ƩDiNP) and negative (driven by ƩDEHTP) associations, which resulted in a negated joint association between the mixture and GWG z-scores in this sub-sample. Our results suggest that women carrying males – more so than those carrying females – may be equally sensitive to chemicals that have opposing effects on GWG. Phthalates/replacements mainly exert their effects on the endocrine system by binding to hormone receptors, such as estrogen receptors alpha and beta that can be found and expressed in different quantities in multiple cell types, which could partially explain different and potentially opposing responses to endocrine disruption (Paterni et al., 2014; Vandenberg et al., 2012). However, additional studies are needed to corroborate these fetal sex-specific mixture findings, as well as to understand what could explain these opposing chemical effects at the physiological level, especially in women carrying males.
Our mixture results in all women are somewhat consistent with those of the Netherlands cohort observing modest inverse associations of urinary phthalic acid (a proxy for total phthalate exposure) with GWG through late pregnancy (Philips et al., 2020). Conversely, using a different method to estimate exposure, the cohort from Anhui Province calculated a hazard index by summing estimated intakes of DBP, BBzP, and DEHP and observed the cumulative index was positively associated with total GWG and odds of excessive total GWG (Gao et al., 2021). Additionally, using BKMR, a study of pregnant women attending a fertility clinic in Boston identified that first trimester DEHP metabolites, MiBP, and propyl paraben contributed most to positive associations between a cumulative mixture of multiple non-persistent chemical classes and total GWG, with DEHP metabolites being the largest contributors (Tyagi et al., 2021). Lastly, the sum of phthalate metabolite biomarkers categorized as anti-androgenic was not associated with total GWG in the Boston women (James-Todd et al., 2016).
As discussed earlier, differences in study characteristics may explain discrepancies between our findings and those from other studies. However, each study, including ours, used different statistical approaches and different proxies of cumulative phthalate exposure or included additional classes of non-persistent chemicals in the mixture, which could also explain inconsistencies across studies. Each statistical mixtures method has its own unique limitations, but also strengths. While a limitation of WQSR is that this method assumes that all chemicals in the mixture are associated with the outcome in the same direction, this method is quite appropriate for estimating the cumulative impact of chemicals from distinct exposure sources (i.e., phthalates/replacements) and identifying the most prominent chemical contributors to this association (Czarnota et al., 2015). In contrast, QGComp is best suited for chemicals from a common exposure source, as it assumes that all exposures are changing in the same direction, not independently; on the other hand, it is able to simultaneously consider chemicals that are associated with the outcome of interest in different directions (Keil et al., 2020). Overall, currently available statistical mixtures approaches have been developed to address a variety of questions that may arise in the field of environmental epidemiology. In the case of associations between phthalates/replacement and GWG, future studies can consider evaluating these relations using the above mentioned and other statistical mixtures methods, including BKMR, which is a robust approach when associations between the chemical mixture and outcome of interest are complex (i.e., interaction, non-linearity) (Bobb et al., 2015).
4.3. Limitations and Strengths
This study has some limitations, but also several strengths. First, we were unable to calculate total GWG, which limits our ability to compare our results to previous studies that considered total GWG or IOM clinical cut-offs. However, we used a previously validated method to calculate GWG z-scores that are standardized by pre-pregnancy BMI and gestational age at late pregnancy weight (Santos et al., 2018), which provides a valid assessment of gestational age- and BMI-specific GWG compared to raw measures alone when total GWG is not available. Second, our choice of using an international GWG reference chart to calculate GWG z-scores may have influenced our observed findings. However, we validated our findings by calculating GWG z-scores using a reference chart developed from a sample of Pittsburgh pregnant women (Hutcheon et al., 2013; Hutcheon et al., 2015), and observed very consistent associations of phthalate/replacement biomarkers with GWG z-scores regardless of the chosen reference chart (data not shown). Third, there are some limitations to using a pooled urine sample to quantify phthalates/replacement metabolite concentrations. We were unable to consider differences by the timing of exposure, which has been demonstrated in other studies (Gao et al., 2021; Philips et al., 2020; Tyagi et al., 2021). Also, we may have lost some temporality for evaluating associations of phthalates/replacements with GWG since our first urine sample was collected towards the end of the first trimester. However, given the non-persistent nature of phthalates/replacements in the body and relatively high within person variability in biomarker concentrations across pregnancy (Shin et al., 2019), pools of up to five first morning urine samples improved the stability of our exposure measure and better approximated total pregnancy exposure to phthalates/replacements compared to a single first morning sample. Fourth, given that I-KIDS is still ongoing, it will take some time to obtain information about the number of women in the analytic sample who developed preeclampsia and gestational diabetes, which could influence observed associations. However, we expect very few cases of pre-eclampsia and gestational diabetes since most women enrolled in I-KIDS did not have major preexisting conditions. Fifth, an important strength is that we evaluated phthalates/replacement biomarkers individually and in a cumulative mixture using both WQSR and QGComp – the latter two allowed us to estimate joint associations of multiple phthalates/replacement biomarkers with GWG z-scores and identify the relative biomarker contributors to these associations. Sixth, while we evaluated many individual-level maternal sociodemographic, lifestyle, and health factors, our results are subject to residual confounding. For example, we did not collect information pertaining to physical activity or sleep quantity/quality before or during pregnancy, which may be important determinants of GWG (Abeysena and Jayawardana, 2011) and urinary phthalate biomarker concentrations (Hatcher et al., 2020; Reeves et al., 2019). There may also be concerns related to co-pollutant confounding. However, we conducted sensitivity analyses limiting our mixture to only include chemicals that crossed the threshold or only include chemicals with positive or negative β-estimates in single pollutant models and observed that all cumulative mixture associations with GWG z-scores remained consistent to what we reported (data not shown). Seventh, studies evaluating GWG typically consider pre-pregnancy BMI as an effect modifier (Hutcheon and Bodnar, 2018), which was not a primary goal of our study because one of our objectives was to focus on fetal sex-specific associations, and we were therefore underpowered to examine associations stratified by fetal sex and pre-pregnancy BMI. However, this is the first study (to our knowledge) to propose and show fetal sex as an important moderator that should be considered in future studies. Lastly, most I-KIDS participants are non-Hispanic white women of relatively high SES, which limits the generalizability of our findings to other populations. However, urinary concentrations of most phthalate and replacement metabolites were similar to those of same age women in the U.S. at similar time periods indicating that exposure in I-KIDS women is consistent with exposure in U.S. women.
5. CONCLUSION
In our relatively high-SES sample of U.S. pregnant women from the Midwest, a mixture of phthalates that included plasticizer replacements DiNCH and DEHTP was marginally associated with lower GWG through late pregnancy. However, our fetal sex-specific findings suggest that women carrying males may be more sensitive to phthalates that may be associated with GWG in opposing directions than those carrying females. Additionally, DEHTP was an important contributor to mixture associations with GWG, which, along with our studies showing that DEHTP is increasing in our sample (Pacyga et al., 2022) and is associated with maternal hormonal disruption (Pacyga et al., 2021), highlights a potential concern for regrettable chemical substitution. Therefore, further research related to this plasticizer replacement is needed in pregnant populations with particular consideration for fetal sex. Finally, experimental animal studies may help elucidate the biological mechanisms underlying the interaction of phthalates/replacement, fetal sex, and GWG.
Supplementary Material
Funding sources:
This publication was made possible by the National Institute for Environmental Health Sciences (NIH/NIEHS) grants ES024795, ES032227, ES022848, the U.S. Environmental Protection Agency grant RD83543401, and National Institute of Health Office of the Director grant UHOD023272. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA or NIH. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. This project was also supported by the USDA National Institute of Food and Agriculture and Michigan AgBioResearch.
Abbreviations:
- AHEI-2010
Alternative Healthy Eating Index
- BBzP
benzylbutyl phthalate
- BKMR
Bayesian Kernel Machine Regression
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- DAG
directed acyclic graph
- DEHP
di(2-ethylhexyl) phthalate
- DEHTP
di(2-ethylhexyl) terephthalate
- DEP
diethyl phthalate
- DiNCH
cyclohexane-1,2-dicarboxylic acid diisononyl ester
- FFQ
Food Frequency Questionnaire
- GWG
gestational weight gain
- GWGz
gestational weight gain z-scores
- I-KIDS
Illinois Kids Development Study
- IOM
Institute of Medicine
- IQR
interquartile range
- MBP
monobutyl phthalate
- MBzP
monobenzyl phthalate
- MCNP
monocarboxynonyl phthalate
- MCOCH
cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester
- MCOP
monocarboxyoctyl phthalate
- MCPP
mono-(3-carboxypropyl) phthalate
- MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- MECPTP
mono(2-ethyl-5-carboxypentyl) terephthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEHHTP
mono(2-ethyl-5-hydroxyhexyl) terephthalate
- MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- MEP
monoethyl phthalate
- MHBP
mono-hydroxybutyl phthalate
- MHiBP
mono-hydroxy-isobutyl phthalate
- MHiNCH
cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester
- MiBP
mono-isobutyl phthalate
- MiNP
monoisononyl phthalate
- MONP
monooxononyl phthalate
- NHANES
National Health and Nutrition Examination Survey
- SES
socioeconomic status
- ƩDBP
sum of di-n-butyl phthalate metabolites
- ƩDEHP
sum of di(2-ethylhexyl) phthalate metabolites
- ƩDEHTP
sum of di(2-ethylhexyl) terephthalate metabolites
- ƩDiBP
sum of di-iso-butyl phthalate metabolites
- ƩDiNCH
sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites
- ƩDiNP
sum of di(isononyl) phthalate metabolites
- US
United States
- WQSR
weighted quantile sum regression
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Statement of ethics: Dr. Joseph M. Braun served as an expert witness in litigation related to perfluorooctanonic acid contamination in drinking water in New Hampshire. Any funds he received from this arrangement were/are paid to Brown University and cannot be used for his direct benefit (e.g., salary/fringe, travel, etc.). No other authors report any conflicts of interest.
REFERENCES
- Abeysena C, Jayawardana P. Sleep deprivation, physical activity and low income are risk factors for inadequate weight gain during pregnancy: a cohort study. J Obstet Gynaecol Res 2011; 37: 734–40. [DOI] [PubMed] [Google Scholar]
- Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019; 567: 305–307. [DOI] [PubMed] [Google Scholar]
- Bellavia A, Hauser R, Seely EW, Meeker JD, Ferguson KK, McElrath TF, et al. Urinary phthalate metabolite concentrations and maternal weight during early pregnancy. Int J Hyg Environ Health 2017; 220: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015; 16: 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Cartus AR, Parisi SM, Abrams B, Himes KP, Eckhardt CL, et al. Pregnancy weight gain in twin gestations and maternal and child health outcomes at 5 years. Int J Obes (Lond) 2021; 45: 1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Siega-Riz AM. A Diet Quality Index for Pregnancy detects variation in diet and differences by sociodemographic factors. Public Health Nutr 2002; 5: 801–9. [DOI] [PubMed] [Google Scholar]
- Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr 2006; 9: 84–93. [DOI] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ Health Perspect 2016; 124: A6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TT, Giovanoulis G, Cousins AP, Magner J, Cousins IT, de Wit CA. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci Total Environ 2016; 541: 451–467. [DOI] [PubMed] [Google Scholar]
- Campioli E, Lee S, Lau M, Marques L, Papadopoulos V. Effect of prenatal DINCH plasticizer exposure on rat offspring testicular function and metabolism. Scientific Reports 2017; 7: 11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environmental health perspectives 2016; 124: 1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 2015; 20: 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield LE, Witter FR, Stoltzfus RJ. Determinants of gestational weight gain outside the recommended ranges among black and white women. Obstet Gynecol 1996; 87: 760–6. [DOI] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012; 142: 1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior 1983; 24: 385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson GM. Perceived Stress in a Probability Sample of the United-States. Social Psychology of Health 1988: 31–67. [Google Scholar]
- Czarnota J, Gennings C, Colt JS, De Roos AJ, Cerhan JR, Severson RK, et al. Analysis of Environmental Chemical Mixtures and Non-Hodgkin Lymphoma Risk in the NCI-SEER NHL Study. Environ Health Perspect 2015; 123: 965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher BG, Frederiksen H, Andersson AM, Juul A, Thankamony A, Ong KK, et al. Serum Phthalate and Triclosan Levels Have Opposing Associations With Risk Factors for Gestational Diabetes Mellitus. Front Endocrinol (Lausanne) 2018; 9: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruh V, Claus Henn B, Weuve J, Wesselink AK, Orta OR, Heeren T, et al. Incidence of uterine leiomyoma in relation to urinary concentrations of phthalate and phthalate alternative biomarkers: A prospective ultrasound study. Environ Int 2021; 147: 106218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher DJ, Hart K, Kotecha S. Common respiratory conditions of the newborn. Breathe (Sheff) 2016; 12: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Tong J, Zhu BB, Geng ML, Gan H, Sun L, et al. Sex-specific mediation of placental inflammatory biomarkers in the effects of prenatal phthalate coexposure on preschooler cognitive development. Environ Sci Pollut Res Int 2022; 29: 13305–13314. [DOI] [PubMed] [Google Scholar]
- Gao H, Zhu BB, Huang K, Zhu YD, Yan SQ, Wu XY, et al. Effects of single and combined gestational phthalate exposure on blood pressure, blood glucose and gestational weight gain: A longitudinal analysis. Environ Int 2021; 155: 106677. [DOI] [PubMed] [Google Scholar]
- Gaston SA, Birnbaum LS, Jackson CL. Synthetic Chemicals and Cardiometabolic Health Across the Life Course Among Vulnerable Populations: a Review of the Literature from 2018 to 2019. Curr Environ Health Rep 2020; 7: 30–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med 2018; 16: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. Executive Summary to EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 2015; 36: 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum A, Dudenhausen J, Skupski DW. Impact of fetal gender on maternal weight gain during pregnancy. American Journal of Obstetrics and Gynecology 2014; 210: S118–S118. [Google Scholar]
- Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. Future Child 1995; 5: 176–96. [PubMed] [Google Scholar]
- Haggerty DK, Upson K, Pacyga DC, Franko JE, Braun JM, Strakovsky RS. REPRODUCTIVE TOXICOLOGY: Pregnancy exposure to endocrine disrupting chemicals: implications for women's health. Reproduction 2021; 162: F169–F180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher KM, Smith RL, Chiang C, Li Z, Flaws JA, Mahoney MM. Association of phthalate exposure and endogenous hormones with self-reported sleep disruptions: results from the Midlife Women's Health Study. Menopause 2020; 27: 1251–1264. [DOI] [PubMed] [Google Scholar]
- Hlisnikova H, Petrovicova I, Kolena B, Sidlovska M, Sirotkin A. Effects and Mechanisms of Phthalates' Action on Reproductive Processes and Reproductive Health: A Literature Review. Int J Environ Res Public Health 2020; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon JA, Bodnar LM. Good Practices for Observational Studies of Maternal Weight and Weight Gain in Pregnancy. Paediatr Perinat Epidemiol 2018; 32: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr 2013; 97: 1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. Pregnancy weight gain charts for obese and overweight women. Obesity (Silver Spring) 2015; 23: 532–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins F, Abrams B, Brooks M, Colvin A, Moore Simas T, Rosal M, et al. The Effect of Gestational Weight Gain Across Reproductive History on Maternal Body Mass Index in Midlife: The Study of Women's Health Across the Nation. J Womens Health (Larchmt) 2020; 29: 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Chiu YH, Messerlian C, Minguez-Alarcon L, Ford JB, Keller M, et al. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ Health 2018; 17: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, et al. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environment International 2016; 96: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A qgcomp: Quantile G-Computation, 2022, pp. R package version 2.8.6. https://CRAN.R-project.org/package=qgcomp. [Google Scholar]
- Keil AP, Buckley JP, O'Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect 2020; 128: 47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraia BA, Bodnar LM, Siega-Riz AM. Pregravid body mass index is negatively associated with diet quality during pregnancy. Public Health Nutr 2007; 10: 920–6. [DOI] [PubMed] [Google Scholar]
- Lau EY, Liu J, Archer E, McDonald SM, Liu J. Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. J Obes 2014; 2014: 524939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Kim S, Bastiaensen M, Malarvannan G, Poma G, Caballero Casero N, et al. Exposure to organophosphate esters, phthalates, and alternative plasticizers in association with uterine fibroids. Environ Res 2020; 189: 109874. [DOI] [PubMed] [Google Scholar]
- Lemke N, Murawski A, Lange R, Weber T, Apel P, Debiak M, et al. Substitutes mimic the exposure behaviour of REACH regulated phthalates - A review of the German HBM system on the example of plasticizers. Int J Hyg Environ Health 2021; 236: 113780. [DOI] [PubMed] [Google Scholar]
- Lessmann F, Schutze A, Weiss T, Bruning T, Koch HM. Determination of metabolites of di(2-ethylhexyl) terephthalate (DEHTP) in human urine by HPLC-MS/MS with on-line clean-up. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1011: 196–203. [DOI] [PubMed] [Google Scholar]
- Li J, Qian X, Zhao H, Zhou Y, Xu S, Li Y, et al. Determinants of exposure levels, metabolism, and health risks of phthalates among pregnant women in Wuhan, China. Ecotoxicology and Environmental Safety 2019a; 184: 109657. [DOI] [PubMed] [Google Scholar]
- Li J, Qian X, Zhao H, Zhou Y, Xu S, Li Y, et al. Determinants of exposure levels, metabolism, and health risks of phthalates among pregnant women in Wuhan, China. Ecotoxicol Environ Saf 2019b; 184: 109657. [DOI] [PubMed] [Google Scholar]
- Li N, Liu E, Guo J, Pan L, Li B, Wang P, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One 2013; 8: e82310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R, Gaskins AJ, Racowsky C, Mansur A, Adir M, Baccarelli AA, et al. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ Int 2018; 111: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mando C, Calabrese S, Mazzocco MI, Novielli C, Anelli GM, Antonazzo P, et al. Sex specific adaptations in placental biometry of overweight and obese women. Placenta 2016; 38: 1–7. [DOI] [PubMed] [Google Scholar]
- Maradonna F, Carnevali O. Lipid Metabolism Alteration by Endocrine Disruptors in Animal Models: An Overview. Front Endocrinol (Lausanne) 2018; 9: 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol 2010; 202: 574 e1–8. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002; 76: 1261–71. [DOI] [PubMed] [Google Scholar]
- Mohammadi H, Ashari S. Mechanistic insight into toxicity of phthalates, the involved receptors, and the role of Nrf2, NF-kappaB, and PI3K/AKT signaling pathways. Environ Sci Pollut Res Int 2021; 28: 35488–35527. [DOI] [PubMed] [Google Scholar]
- Muscogiuri G, Barrea L, Laudisio D, Savastano S, Colao A. Obesogenic endocrine disruptors and obesity: myths and truths. Arch Toxicol 2017; 91: 3469–3475. [DOI] [PubMed] [Google Scholar]
- Neier K, Cheatham D, Bedrosian LD, Dolinoy DC. Perinatal exposures to phthalates and phthalate mixtures result in sex-specific effects on body weight, organ weights and intracisternal A-particle (IAP) DNA methylation in weanling mice. J Dev Orig Health Dis 2019; 10: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga DC, Gardiner JC, Flaws JA, Li Z, Calafat AM, Korrick SA, et al. Maternal phthalate and phthalate alternative metabolites and urinary biomarkers of estrogens and testosterones across pregnancy. Environ Int 2021; 155: 106676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga DC, Haggerty DK, Nicol M, Henning M, Calafat AM, Braun JM, et al. Identification of profiles and determinants of maternal pregnancy urinary biomarkers of phthalates and replacements in the Illinois Kids Development Study. Environ Int 2022; 162: 107150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatianou ME, Simos YV, Andronikou SK, Kiortsis DN. Long-term metabolic effects of high birth weight: a critical review of the literature. Horm Metab Res 2014; 46: 911–20. [DOI] [PubMed] [Google Scholar]
- Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERalpha) and beta (ERbeta): subtype-selective ligands and clinical potential. Steroids 2014; 90: 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Santos S, Steegers EAP, Asimakopoulos AG, Kannan K, Trasande L, et al. Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environment International 2020; 135: 105342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh SJ, Richardson GA, Hutcheon JA, Himes KP, Brooks MM, Day NL, et al. Maternal Obesity and Excessive Gestational Weight Gain Are Associated with Components of Child Cognition. J Nutr 2015; 145: 2562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Shao H, Ying X, Huang W, Hua Y. The Endocrine Disruption of Prenatal Phthalate Exposure in Mother and Offspring. Front Public Health 2020; 8: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzetti SC P; Just AC; Bello G; Gennings C gWQS: Generalized Weighted Quantile Sum Regression, 2021, pp. R package version 3.0.4. https://CRAN.R-project.org/package=gWQS. [Google Scholar]
- Rasmussen KM, Yaktine AL. Weight Gain During Pregnancy: Reexamining the Guidelines, Washington (DC), 2009. [PubMed] [Google Scholar]
- Reeves KW, Santana MD, Manson JE, Hankinson SE, Zoeller RT, Bigelow C, et al. Predictors of urinary phthalate biomarker concentrations in postmenopausal women. Environ Res 2019; 169: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Carmona Y, Ashrap P, Calafat AM, Ye X, Rosario Z, Bedrosian LD, et al. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J Expo Sci Environ Epidemiol 2020; 30: 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos S, Eekhout I, Voerman E, Gaillard R, Barros H, Charles MA, et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med 2018; 16: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer RM, Ferguson KK, Sheppard L, James-Todd T, Butts S, Chandrasekaran S, et al. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ Int 2019; 123: 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HM, Bennett DH, Barkoski J, Ye X, Calafat AM, Tancredi D, et al. Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ Int 2019; 122: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Jia T, Samandar E, Preau JL Jr., Calafat AM. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000-2012). Environ Res 2013; 126: 159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr., Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 860: 106–12. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Wong LY, Samandar E, Preau JL Jr., Jia LT, Calafat AM. Exposure to di-2-ethylhexyl terephthalate in the U.S. general population from the 2015-2016 National Health and Nutrition Examination Survey. Environ Int 2019; 123: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomro MH, Maesano CN, Heude B, Bornehag CG, Annesi-Maesano I. The association between maternal urinary phthalate metabolites concentrations and pregnancy induced hypertension: Results from the EDEN Mother-Child Cohort. J Gynecol Obstet Hum Reprod 2021; 50: 102216. [DOI] [PubMed] [Google Scholar]
- Springel EH, Berggren EK, Huston-Presley L, Catalano PM. What is the impact of fetal sex on maternal glucose metabolism? American Journal of Obstetrics and Gynecology 2016; 214: S307–S307. [Google Scholar]
- Strakovsky RS, Schantz SL. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ Epigenet 2018a; 4: dvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Schantz SL. Using Experimental Models to Assess Effects of Bisphenol A (BPA) and Phthalates on the Placenta: Challenges and Perspectives. Toxicol Sci 2018b; 166: 250–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succop PA, Clark S, Chen M, Galke W. Imputation of data values that are less than a detection limit. J Occup Environ Hyg 2004; 1: 436–41. [DOI] [PubMed] [Google Scholar]
- Tanner EM, Bornehag CG, Gennings C. Repeated holdout validation for weighted quantile sum regression. MethodsX 2019; 6: 2855–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torniainen M, Wegelius A, Tuulio-Henriksson A, Lonnqvist J, Suvisaari J. Both low birthweight and high birthweight are associated with cognitive impairment in persons with schizophrenia and their first-degree relatives. Psychol Med 2013; 43: 2361–7. [DOI] [PubMed] [Google Scholar]
- Tyagi P, James-Todd T, Minguez-Alarcon L, Ford JB, Keller M, Petrozza J, et al. Identifying windows of susceptibility to endocrine disrupting chemicals in relation to gestational weight gain among pregnant women attending a fertility clinic. Environ Res 2021; 194: 110638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012; 33: 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Robins JM. Signed directed acyclic graphs for causal inference. J R Stat Soc Series B Stat Methodol 2010; 72: 111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Pu Y, Gingrich J, Padmanabhan V. Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends Endocrinol Metab 2018; 29: 607–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Hu YB, Gao H, Sheng J, Huang K, Zhang YW, et al. Sex-specific difference in placental inflammatory transcriptional biomarkers of maternal phthalate exposure: a prospective cohort study. J Expo Sci Environ Epidemiol 2020; 30: 835–844. [DOI] [PubMed] [Google Scholar]
- Warrington NM, Richmond R, Fenstra B, Myhre R, Gaillard R, Paternoster L, et al. Maternal and fetal genetic contribution to gestational weight gain. Int J Obes (Lond) 2018; 42: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein RL, Lazar NA. The ASA's Statement on p-Values: Context, Process, and Purpose. American Statistician 2016; 70: 129–131. [Google Scholar]
- Weaver JA, Beverly BEJ, Keshava N, Mudipalli A, Arzuaga X, Cai C, et al. Hazards of diethyl phthalate (DEP) exposure: A systematic review of animal toxicology studies. Environ Int 2020; 145: 105848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch BM, Keil AP, Bommarito PA, van T' Erve TJ, Deterding LJ, Williams JG, et al. Longitudinal exposure to consumer product chemicals and changes in plasma oxylipins in pregnant women. Environ Int 2021; 157: 106787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The HOME Study. Environ Health 2015; 14: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect 2011; 119: 878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardo V, Giliberti L, Giliberti E, Grassi A, Perin V, Parotto M, et al. The role of gestational weight gain disorders in symptoms of maternal postpartum depression. Int J Gynaecol Obstet 2021; 153: 234–238. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mustieles V, Yland J, Braun JM, Williams PL, Attaman JA, et al. Association of Parental Preconception Exposure to Phthalates and Phthalate Substitutes With Preterm Birth. JAMA Netw Open 2020; 3: e202159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin H, Eskenazi B, Holland N, Harley KG. Prenatal exposure to phthalates and maternal metabolic outcomes in a high-risk pregnant Latina population. Environ Res 2021; 194: 110712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.