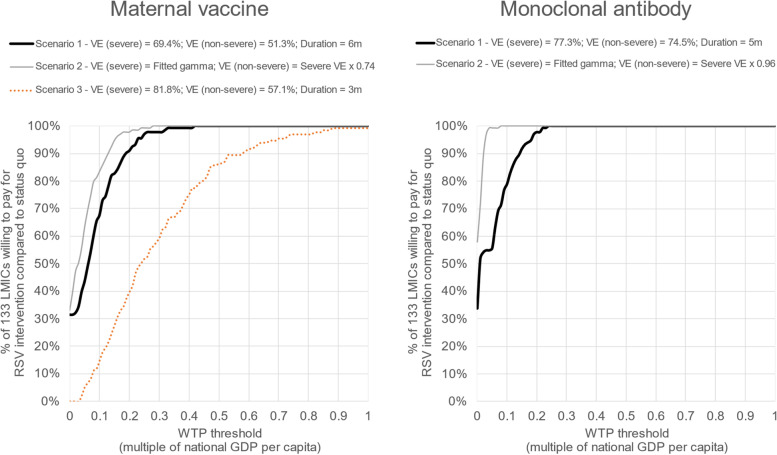
Fig. 3.
Percentage of 133 LMICs willing to pay for RSV prevention strategies (compared to no pharmaceutical intervention) at different willingness-to-pay thresholds: base case and alternative efficacy scenarios. Caption: The thick black lines show the base case (scenario 1) assumptions. For maternal vaccination, this assumes efficacy of 69.4% (severe RSV disease) and 51.3% (non-severe RSV disease) for a 6-month period and zero protection thereafter. For mAb this assumes efficacy of 77.3% (severe RSV disease) and 74.5% (non-severe RSV disease) for a 5-month period, and zero protection thereafter. The grey lines show the scenario 2 assumptions. For this scenario, we used previously described methods [53] to fit estimates of instantaneous efficacy (iE) that were consistent with the reported cumulative efficacy (cE) at 3 and 6 months of follow-up (see Additional file 1, page 32, and Fig. S3, for more details). Finally, we applied one additional scenario (scenario 3) for maternal vaccination with efficacy of 81.8% (severe RSV disease) and 57.1% (non-severe RSV disease) for a 3-month period, and zero protection thereafter