Abstract
Type II Diabetes (T2D) is a major risk factor for Alzheimer’s Disease (AD). These two diseases share several pathological features, including amyloid accumulation, inflammation, oxidative stress, cell death and cognitive decline. The metabolic hormone amylin and amyloid-beta are both amyloids known to self-aggregate in T2D and AD, respectively, and are thought to be the main pathogenic entities in their respective diseases. Furthermore, studies suggest amylin’s ability to seed amyloid-beta aggregation, the activation of common signaling cascades in the pancreas and the brain, and the ability of amyloid beta to signal through amylin receptors (AMYR), at least in vitro. However, paradoxically, non-aggregating forms of amylin such as pramlintide are given to treat T2D and functional and neuroprotective benefits of amylin and pramlintide administration have been reported in AD transgenic mice. These paradoxical results beget a deeper study of the complex nature of amylin’s signaling through the several AMYR subtypes and other receptors associated with amylin effects to be able to fully understand its potential role in mediating AD development and/or prevention. The goal of this review is to provide such critical insight to begin to elucidate how the complex nature of this hormone’s signaling may explain its equally complex relationship with T2D and mechanisms of AD pathogenesis.
Keywords: Alzheimer’s disease (AD), type II diabetes (T2D), amyloid, amyloid beta, amylin, calcitonin receptor, receptor activity modifying protein (RAMP)
1. INTRODUCTION
As the life expectancy of people around the world continues to increase with advances in science and medicine, the prevalence of age-related disorders also increases. Alzheimer’s Disease (AD) is the leading cause of dementia and the sixth leading cause of death in the United States that primarily impacts the elderly population, with the majority of those diagnosed above the age of 65. After 65, the risk of AD development doubles every five years, and reaches nearly 1/3 by the age of 85 (1). While the incidence of the leading cause of death; heart disease, decreased 11% from 2000 to 2015, AD increased 123% over the same time period speaking to the critical relevance to find a therapeutic [1] as this rate is only predicted to keep rising with 13.8 million Americans to be diagnosed by 2050 [2].
AD is a progressive neurodegenerative disease that impairs memory, problem-solving, language and other cognitive abilities [1]. The initial symptoms of AD typically involve episodic memory loss, which eventually progresses to an inability to perform simple tasks. AD patients also undergo a number of behavioral changes, which can include depression, psychosis, executive dysfunction, irritability, sleep disorders and even personality changes [2]. The average duration of the illness is 8–10 years, but the preclinical and prodromal stages that precede the clinical symptomatic stages typically extend over 20 years [3].
There are two classifications of AD - sporadic and familial. Early-onset familial AD occurs in younger subjects, with a mean age of 45, due to an inherited genetic mutation, but accounts for less than 1% of all AD cases [3]. While many genetic mutations are linked to familial AD, the majority of cases stem from amyloid precursor protein (APP), presenilin (PSEN1 or PSEN2) and Apolipoprotein E 4 (APOE4) mutations [4, 5]. These mutations lead to alterations in APP metabolism; and increased production and aggregation of the amyloid beta-peptide (Aß), a hallmark pathological feature of AD [6]. The overwhelming majority of AD cases are sporadic, with a late onset over the age of 65. The cause of late-onset AD is not known, and the pathogenesis involves multiple environmental and genetic factors [1], making prevention and treatment increasingly difficult to pinpoint.
AD is commonly characterized by many features including neurodegeneration, oxidative stress (OS), neuroinflammation, decreased brain metabolism, impaired synaptic transmission and neuronal cell death, as well as two hallmark lesions; extracellular amyloid plaques composed of Aß and intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau [3, 7–11]. Tau is a microtubule-associated protein that becomes hyperphosphorylated and self-aggregates in AD brains, levels of which correlate with cognitive impairment and cell death [7, 12–14].
Aß is a 37–43 amino acid (AA) peptide that is the cleavage product of APP, orchestrated by ß-secretase (BACE1) and subsequent γ-secretase cleavage [7, 15]. While 90% of basal Aß production is cleaved at AA 40 (Aß1–40), cleavage at AA site 42 (Aß1–42) levels are elevated in AD patient brains [16–18]. Mutations to γ-secretase catalytic subunits PSEN1 & PSEN2, as well as APP, lead to the overproduction of Aß1–42 [18–22]. Aß1–42 is more prone to aggregation and is believed to be the building block for the toxic Aß oligomers, which affect memory and cell survival [23]. While there is much debate as to whether Aß oligomers or amyloid plaques are the more toxic species of Aß, it is clear that there is a positive feedback loop established between Aß accumulation and OS, neuroinflammation and cell death mechanisms, contributing to the pathological cascade observed in AD [23–26]. This destructive feed-forward mechanism due to amyloidosis is not restricted to AD but involves other diseases as well, namely Type 2 Diabetes Mellitus (T2D).
2. RELATIONSHIP BETWEEN AD & T2D
Sporadic AD, is the result of numerous genetic and environmental factors [27]. Lifestyle choices such as diet and exercise that can lead to obesity, metabolic syndrome and the development of T2D are associated with sporadic AD development [28–30]. Diabetics who have T2D for more than five years have significantly increased risk for AD development [31]. AD has even been referred to as ‘Type 3 Diabetes’ by some, indicating the strikingly similar pathological features between Diabetes and AD [26, 30, 32], particularly in the brain.
Diabetes is the seventh leading cause of death in the United States, just behind AD. According to the CDC, in 2015, 30.3 million people; 9.4% of the US population, had diabetes and a staggering 84.1 million had prediabetes; 33.9% of the US population. The prevalence of T2D is expected to increase to almost 600 million cases worldwide by the year 2045. While men develop T2D more frequently than women, women develop T2D-associated cognitive decline more commonly than men [33]. Women are also more prone to develop dementia and AD [1], suggesting a unique sex difference in pathogenesis in both diseases.
T2D is a metabolic disease derived from chronic hyperglycemia, typically due to poor diet and a sedentary lifestyle [34], which leads to chronic hyperinsulinemia and hyperamylinemia as the body tries to regulate blood glucose levels [35]. This leads to insulin resistance, impaired insulin signaling in the brain, impaired glucose utilization, and the eventual decrease in insulin and amylin production with the pancreatic ß-cell loss [36].
AD & T2D share many pathological characteristics, which include inflammation, mitochondrial dysfunction, OS and importantly, to this review: decreased brain metabolism, impaired metabolic hormone signaling and resistance, amyloid accumulation and cognitive decline [32, 37, 38]. Overproduction of insulin due to chronic high blood glucose levels causes the downregulation of insulin receptors, impaired transport of insulin across the blood-brain barrier (BBB) and reduced insulin signaling in the brain [10, 39–41]. As insulin’s primary function is to modulate blood glucose levels [42], this leads to impaired cerebral glucose uptake and utilization, which is one of the first signs of cognitive impairment that continues to worsen with cognitive decline [10, 43, 44]. Impaired cerebral glucose utilization leads to neuronal starvation, impaired energy production, mitochondrial dysfunction, OS, DNA damage and increased cell death, all of which drive pro-apoptotic, pro-inflammatory and pro-Aß cascades [45, 46].
Insulin receptors (IR) are widely distributed throughout the brain and are highly expressed in the hippocampus and cerebral cortex, downregulation of which correlates with deficits in synaptic transmission and impaired learning [42, 47]. This is likely due to the various cascades IRs are associated with, including neuronal plasticity, learning and memory, which insulin and insulin-like growth factor (IGF) agonism activate; dysregulation of which contributes to cognitive decline [32, 48, 49]. This is supported by the finding that acute insulin administration was shown to improve performance on memory and cognition tests [50]. Conversely, chronic hyperinsulinemia induced the opposite effect [51], likely due to the development of insulin resistance and impaired insulin signaling.
Impaired insulin signaling also induces increased tau hyperphosphorylation via impaired downstream PI3K-Akt signaling and subsequent dephosphorylation and activation of glycogen synthase kinase 3ß (GSK3ß), which is normally responsible for binding tau to microtubules [52]. The inability to properly inhibit GSK3ß activity leads to the hyperphosphorylation and subsequent aggregation of tau. IR dysfunction has also been shown to impair Aß clearance and contribute to Aß aggregation [53]. This seems to establish a positive feedback loop as Aß has been shown to impair insulin signaling and induce insulin resistance [54, 55]. Dysregulated insulin signaling also leads to impaired Aß clearance via changes in the insulin-degrading enzyme (IDE), as IDE degrades not only insulin but Aß as well [56–58]. Furthermore, IDE activity decreases in both T2D and AD brains, which may also lead to amyloid aggregation [59]. As mentioned above, both AD and T2D pathology include an aggregation of amyloid protein that leads to toxic effects. To be discussed further in this review, amylin has also been implicated in AD and may be an important player in both diseases.
3. AMYLIN & THE AMYLIN RECEPTOR
Amylin is a 37-AA peptide hormone that is produced in the ß-cells of the pancreatic islets of Langerhans. Amylin is co-produced and co-secreted from the pancreas with insulin after eating [60]. Amylin works in conjunction with insulin to reduce blood glucose by slowing gastric and intestinal emptying, inhibiting glucagon secretion and reducing food intake [61]. Amylin has both paracrine and endocrine function. It is important to note that this hormone is readily BBB permeable and amylin receptor (AMYR) function has been found throughout the CNS, suggesting the potential relevance and importance of amylin signaling in the healthy brain within multiple systems.
The AMYR is composed of two major components; the calcitonin receptor (CalcR) and receptor activity modifying protein (RAMP). The CalcR is a G-protein coupled receptor (GPCR) that localizes to the cell surface [62, 63]. The bone-dwelling osteoclast is the primary target of calcitonin (CT), but receptors have been reported throughout the periphery in organs such as the kidney, lung, testes, placenta and skeletal muscle [64, 65]. It has also been reported to be widely distributed in the CNS. Huang et al., detected CalcR mRNA in the spinal cord and various regions of the mouse brain, including the nucleus accumbens (NAc), cortex, hippocampus and hypothalamus. Immunohistochemical analysis has located CalcR protein expression in the area postrema (AP), the NAc, a number of hypothalamic nuclei, the substantia nigra, stria terminalis, locus coeruleus, nucleus of the solitary tract (NTS) and some nuclei of the reticular formation [66]. Many of these findings were found to be consistent in human brains, as well [67].
Several splice variants of the CalcR have been discovered, which is further complicated by variation across species [68]. The CalcRα isoform is the most abundant and widely studied CalcR isoform. CalcRß is the next most common isoform of the CalcR, which is identical to CalcRα, except for the addition of a 16-amino-acid insert in the first intracellular domain. [69]. There has been little difference observed between the two receptor isoforms in the peptide ligands they respond to, although the affinity to each ligand and ability to activate downstream pathways varies depending on the splice variant and cellular background. Two other splice variants of the CalcR have been implicated, but these transcripts correspond to uncommon or non-functional proteins [69].
The CalcR signals primarily for CT, which has many functions, but is most widely known for blood calcium level regulation by osteoclast-mediated bone resorption inhibition and renal calcium clearance stimulation [68]. In addition to CT, the CalcR interacts with the Calcitonin Gene-Related Peptide (CGRP) and adrenomedullin (AM), resulting primarily in vasodilation [62]. The CalcR has also been shown to respond to amylin in several different cell lines, but its affinity for each of the latter three ligands is greatly decreased when compared to CT. The affinity of the CalcR for amylin is greatly increased when complexed to a RAMP [61, 62, 70].
To date, three separate RAMPs have been identified; RAMP1, RAMP2 and RAMP3. Despite sharing a common structure and similar functions, RAMP proteins only have 30% sequence homology. RAMP2 is the longest isoform at 174 amino acids, while RAMPs 1 and 3 are 148 amino acids long [71]. Similar to the CalcR, mRNA and protein expression of all RAMP subtypes has been reported to be widely distributed throughout a wide variety of human and rodent peripheral tissues. As seen in the NCBI protein atlas, there is pronounced expression of RAMP1 mRNA as well as protein in the endometrium, prostate, pancreas and muscle tissue, RAMP2 in the lung, placenta, adipose tissue and muscle tissue, and RAMP3 in the lung, lymph nodes and thyroid gland observed in humans [72]. The expression of mRNA of all three RAMPs is widely distributed among peripheral organs in rodents as well [72]. To date, there has only been RAMP protein expression observed in the human cortex, as described in the NCIB protein atlas. More extensive studies have been conducted on RAMP expression in the rodent brain, indicating that each of the three RAMP subtypes are widely distributed throughout the brain, including expression in the AP, subfornical organ (SFO), hypothalamus, ventral tagmental area (VTA), hippocampus, cerebellum and a wide variety of hypothalamic nuclei. Additionally, RAMP1 expression has been observed in the cerebral cortex, caudate putamen, amygdaloid complex and NAc, RAMP2 in the NTS, pia mater and blood vessels, and RAMP3 in the cerebral cortex [73–76].
While one, two or all three RAMP proteins have been shown to interact with eleven different GPCRs, it is only their interaction with the CalcR that results in amylin signaling [77]. The interaction of RAMP1, RAMP2 and RAMP3 with the two CalcR isoforms, CalcRα and CalcRß, result in the six separate AMYRs: AMYR1α, AMYR2α, AMYR3α, AMYR1ß, AMYR2ß and AMYR3ß, respectively. AMYR1α, & AMYR3α have received the brunt of the experimental attention. While the stoichiometry and biochemistry of this interaction have not yet been investigated, numerous studies have generated functional results using a 1:1 CalcR:RAMP ratio.
Interestingly, multiple studies have shown all AMYR subtypes in addition to each splice variant of the CalcR to have the highest affinity for salmon CT (sCT) in multiple cell lines. When RAMPs 1 & 3 interact with either CalcR splice variant, in COS-7, CHO-P, and RAEC cells, the affinity for amylin increases while simultaneously decreasing CT affinity [62, 70, 78]. Christopoulos, et al. demonstrated that transfection of increasing levels of RAMPs 1 and 3 into CHO-K1 cells, which endogenously express CalcR, increased amylin binding as well. Generally speaking, these studies indicate that AMYR1 receptors have the highest affinity for sCT, followed by amylin, CGRP, CT, and then AM. The AMYR3 receptor shares similar affinities for these ligands, although the affinity for CGRP is markedly reduced when compared to AMYR1.
There are mixed results, however, on the impact of RAMP2 co-expression with the CalcR on amylin binding, Muff, et al., and Lee et al., indicate an increase in amylin potency in RAEC cells [78] and HEK cells [79], respectively. Hay et al., indicate no increase in amylin affinity in response to AMYR2 expression in COS7 cells [61], which agrees with the results indicated by Christopoulos et al., [62]. Morfis et al., on the other hand, indicate that AMYR2-transfected COS7 cells exhibit an increased amylin potency, roughly equal to that of the AMYR1 and AMYR3 receptors [80]. Christopoulos et al. also indicated that RAMP2 did not influence amylin binding in CHO-K1 cells, and that only RAMP1 co-transfection with the CalcR was able to alter amylin signaling in HEK cells. These results further indicate the importance of cellular background on AMYR function, along with the importance of experimental consistency across studies – particularly in regard to transfection and binding assay procedures. While it has become generally accepted that AMYR1 and AMYR3 are the more prominent receptors involved in amylin signaling, these results indicate that further study is necessary to fully understand the role of RAMP2 and AMYR2 in amylin binding and signaling.
Amylin has also been shown to signal through the calcitonin receptor-like receptor (CRLR) when it is complexed to a RAMP, but at much lower affinities. When the CRLR is complexed with RAMP1, it acts primarily as a CGRP receptor, but it has also been shown to respond to AM, Adrenomedullin 2/Intermedin (AM2), amylin and CT, in that order of potency. The AM receptor is composed of the CRLR and either RAMP2 or RAMP3, resulting in the AM1 and AM2 receptors, respectively. AM1 responds to AM, amylin, CGRP, AM2 and CT, in that order. The AM2 responds to each peptide with similar affinities, but amylin and AM2 are switched in the list of affinities when compared to AM1 [63, 69, 81].
Another receptor has recently been implicated in amylin signaling in addition to the traditional RAMP/CalcR complex; the transient receptor potential cation channel subfamily V member 4 (TRPV4) receptor. The NIH Genetics Home reference shows that TRPV4 receptor is a versatile nonselective cation channel activated by osmotic, mechanical and chemical cues that plays a role in a wide array of physiological functions. More recently, it has been suggested to play a role in amylin signaling, particularly at toxically high concentrations of amylin; concentrations at which amylin has been shown to aggregate [82, 83]. The affinity of amylin to the TRPV4 receptor has not yet been investigated. The TRPV4 receptor exhibits high levels of protein expression throughout the human periphery, with enhanced expression in the adrenal gland, pancreas, gastrointestinal tract and genitalia (protein atlas). The NCIB protein atlas, Kauer, 2009 and Zhang, 2017 describe that TRPV4 receptor is likewise widely expressed throughout the brain in humans and rodents, including the hippocampus, cortex, cerebellum and caudate putamen [83, 84].
Specific peripheral amylin binding has been reported in the rodent lung, stomach fundus, spleen, and liver [85]. Moderate to high amylin binding in the rodent brain has been observed in the mid-caudal NAc, the fundus striati, the bed nucleus of the stria terminalis, the substantia inominata, the amygdalostriatal transition zone, the central and medial amygdalostriatal nuclei, a number of hypothalamic nuclei, locus coeruleus, dorsal raphe and caudal parts of the NST. The highest amount of amylin binding was observed in the SFO, lamina terminalis and the AP [86]. More recent studies have suggested amylin binding in the hippocampus [83, 87–89].
In addition to what is known about amylin’s signaling, there have been scattered reports suggesting amylin production in the brain. Two reports covered later in this review found increased levels of amylin mRNA and protein in the preoptic area, medial preoptic nucleus and bed nucleus of the stria terminalis of postpartum rat dams [90, 91]. Amylin immunoreactive cell bodies were found in various regions of the rat brainstem [92] and various monkey hypothalamic nuclei [93]. mRNA levels of proislet amyloid polypeptide (proIAPP), the precursor to amylin, were also located in various nuclei in the mouse hypothalamus. However, the relevance of central amylin production has not yet been investigated.
4. AMYLIN & ITS RELATIONSHIP TO AD & COGNITION
In normal conditions, hAmy exists as a soluble monomer, but undergoes conformational and biochemical changes in T2D, leading to aggregation and fibril formation [94]. These amylin fibrils, which are found in the brain and pancreas of over 90% of T2D patients, are closely linked with pancreatic ß-cell death, and the consequential decrease in amylin and insulin production during late-stage T2D [36, 95, 96]).
Amylin not only aggregates in the pancreas and causes ß-cell death, but it also readily crosses the BBB and aggregates in the brain, leading to cognitive impairment [97, 98]. While amylin and AMYR expression in the healthy brain has been mentioned throughout the article thus far, unfortunately, studies searching for amylin and AMYR distribution in AD brains have yet to be done. To this end, Aß and amylin aggregates, as well as mixed plaques consisting of both amyloid proteins, have all been found in AD brains. Fibrillar amylin has been suggested to seed Aß aggregation and plaque formation, while monomeric amylin may inhibit Aß aggregation [99, 100]. Whether amylin in itself is a toxic insult, or whether its functional loss via aggregation and ß-cell loss in T2D participates in AD development is still a topic of debate. This is highlighted by several conflicting articles supporting benefits of both AMYR agonists and antagonists [37].
Due to its multifactorial nature, drug development for AD therapy has proven to be a challenge, with no new treatment approvals since 2003. Currently, the six drugs approved by the FDA for AD and dementia treatment only beginning to address symptomatic effects of the disease rather than begin to be preventative in nature [101, 102]. As mentioned early in this review, dysfunction in brain metabolism, inflammation and the production of reactive oxidative species is known to be present in the brain years before the first behavioral signs of memory loss are present. Therefore, there is a critical need to find biomarkers that elucidate the time-point when therapeutic intervention needs to be administered, most likely before the point of Aß aggregation. Recent evidence, however, has shown promise in the use of pramlintide acetate (PRAM) as a therapeutic for AD in AD model mice. PRAM, a synthetic analogue of amylin, was synthesized to mimic non-aggregating rat amylin (rAmy), which differs from hAmy by three proline substitutions at positions 25, 28 and 29, the AAs responsible for amyloid formation [103]. Studies have also indicated PRAM’s ability to slow hAmy aggregation in vitro [104]. Restoring amylin levels in the form of non-aggregating PRAM, in diabetic patients has shown promising evidence that it may be the loss of functional amylin in the brain, along with insulin, that may be the underlying cause of cognitive dysfunction in T2D. This theory is further supported by findings indicating a positive association between levels of plasma amylin and cognition in healthy elderly subjects, T2D patients and AD patients [105–107]. This serum relationship alone, paired with the fact that AMYR mRNA is expressed in areas related to AD pathology and higher-order cognition, i.e., the hippocampus and cortices, gives sufficient rationale to look at the role of amylin in healthy cognition, memory and its potential relation to the dysfunction of those processes in AD pathogenesis. As hypometabolism in the brain of T2D and AD patients has already been mentioned many times in this review, it should not be a surprise that hypothesizing hyperamylinemia along with hyperinsulinemia due to insulin resistance, and possibly amylin resistance even though those studies are yet to be done, during late-stage T2D can leading to aggregates of both amylin and Aß that downstream cause cognitive decline is a loss-of-function theory that could be addressed by hormone replacement.
The results of numerous animal studies also support the theory of therapeutic amylin replacement. To this end, several studies have demonstrated the benefits of centrally and peripherally administered PRAM on memory and cognition in various animal models of AD. Subcutaneously PRAM treated senescence-accelerated prone (SAMP8) mice performed significantly better at the hippocampal-dependent novel object recognition task than saline-treated mice [107]. This was in conjunction with increases in both synapsin I and CDK5, two proteins implicated in synapse growth and formation. This same study indicated that PRAM also exhibited antioxidant and anti-inflammatory effects as PRAM treated mice showed a significant decrease in the oxidative stress marker HO-1, and the inflammation marker, COX-2 [107]. More recently, Patrick et al. indicated that chronic peripheral treatment of PRAM in APP/PS1 mice increased OS handling machinery. In addition, PRAM was able to dose-dependently reduce ROS induced by an H2O2 insult to neuroblastomas, in vitro [108]. These findings suggest non-aggregating amylin may act as an antioxidant or activate down-stream defensive antioxidants.
Intraperitoneal (IP) treatment of PRAM and hAmy improved both Y maze and Morris water maze performance, and both IP and intracerebroventricular (ICV) treatment reduced pathology and increased Aß1–42 in the CSF of 5XFAD AD model mice; suggesting that amylin may function to shuttle Aß out of the brain [109]. IP injections of hAmy were also shown to reduce tau pathology in 3xTg AD mice and neuroinflammation markers Iba-1 and CD68, while simultaneously improving cognition [110]. Importantly, this study also indicated that IP injection of the AMYR inhibitor, AC253, attenuated the beneficial effects observed on cognition and pathology, indicating the importance of amylin interaction with its cognate receptor to mediate these therapeutic effects.
Other studies, however, have indicated the therapeutic potential of AMYR inhibition in vivo and in vitro. Treatment of TgCRND8 mice with AC253 and a cyclic form of AC253, (cAC253), decreased plaque burden and neuroinflammation while improving cognition [87]. The same study indicated that both AC253 and cAC253 attenuated cell death induced by hAmy and Aß1–42 in HEK cells overexpressing AMYR3 [87]. In vitro, both hAmy and Aß exerted dose-dependent neurotoxic effects when administered to primary hippocampal, cortical and forebrain cholinergic neurons [111, 112]. Similar studies also indicated impaired cell and neuronal viability [74, 113], as well as increases in apoptotic markers caspases 3, 6, 9, BID and XIAP, by both Aß and hAmy [114]. Soluble Aß1–42 oligomers and hAmy also induced impairments in LTP in hippocampal slices of TgCRND8 AD mice. These effects were blocked by AC253, but interestingly, by PRAM as well [88, 89]. Given that PRAM did not exert an effect on LTP when administered alone, the authors postulated that PRAM exerts its therapeutic effects by way of blocking the toxic actions of amylin and Aß [88]. Together, the studies summarized above seem to highlight a discrepancy between the results of in vivo and ex vivo/in vitro studies and potentially in the nature of how different agonists and antagonists bind and signal through the AMYR. It is a possibility that amylin signaling via AMYR elicits different signaling cascades dependent on cellular subtype, AMYR complex present, the genetic background of a mouse model and dose of hAMY/PRAM/AMYR antagonist used in each experiment. More work in this area of amylin functionality is warranted in order to truly understand the pharmokinetics of activation versus inhibition of the AMYR.
4.1. AMYR3 Prevalence in Cognition & Aß Signaling
Morfis et al. conducted an in-depth analysis of signaling pathways activated by amylin. HEK cells and COS7 cells were transfected with the CalcRα and either RAMP1, RAMP2 or RAMP3 and subsequently treated with rAmy. The cellular response results compared to that of human CT. They observed a 20 – 40-fold increase in cAMP signaling, a 2 – 5-fold increase in ERK phosphorylation, both of which are known to be important signaling pathways involved in cognition. Morfis et al. also indicated a 2 – 4-fold increase in intracellular calcium depending upon the RAMP isoform and cell type [80]. With the exception of the cAMP response in COS7 cells, which showed a more potent response in cells transfected with RAMP1, all dose-response assays indicated a more potent response in cells transfected with RAMP3 [80].
Based on the results of the experiments conducted by Morfis et al. indicating a prominent role for AMYR3, Fu et al. investigated the signaling cascades mediated by that particular receptor. HEK cells transfected with AMYR3 displayed an increase in cytosolic cAMP and calcium, as well as an increase in Akt, ERK and PKA activity; as indicated by phosphorylation, upon receptor activation [113]. The role of AMYR3 was confirmed when AC253 was shown to block the increase in intracellular calcium, as well as activation of PKA, Akt and ERK. The importance of AMYR3 in mediating amylin (and Aß) effects was supported by findings indicating that knockdown of the CalcR and RAMP3 rendered human fetal neurons more resistant to hAmy and Aß induced cell death [74].
Several studies have indicated that amylin and Aß signal through the same receptor. Studies mentioned above indicated that specific AMYR inhibition attenuated increased cell death [74, 87, 112, 113], decreased levels of pro-apoptotic mediators, and rescued impaired LTP [88, 89] induced by both hAmy and Aß. Aß1–42 was also shown to induce increases in intracellular cAMP and calcium as well as activation of Akt, ERK and PKA in AMYR3 expressing HEK cells, similar to hAmy [113]. Co-application of hAmy and Aß1–42 did not increase the neuronal toxicity [111] or the rise of cytosolic calcium [113] observed when compared to incubation of a single peptide, indicating a common receptor and/or signaling pathway. Gingell et al., however, indicated that Aß1–42 was unable to evoke a cAMP response through HEK and COS7 cells overexpressing the AMYR1 or AMYR3 receptors at a wide variety of concentrations, when hAmy, rAmy and PRAM all induced a significant increase of cytosolic cAMP through both receptor subtypes [115]. As the same receptor subtype, cellular background and Aß isoform were used for both studies, the cause of the discrepancy of results regarding the ability of Aß to stimulate cAMP production is unclear [113, 115].
4.2. TRPV4 & Calcium Signaling: Relevance to Excitotoxicity in AD
As the TPRV4 receptor is a nonselective cation channel expressed widely throughout the periphery and the brain, its functions are numerous. These functions include that of amylin signaling, although this signaling cascade has not been studied as extensively. Low concentrations of hAmy, rAmy and PRAM were shown to induce small but significant increases in intracellular calcium in primary hippocampal neurons, which was blocked by specific AMYR inhibition [83]. At high concentrations of hAmy; where amylin was shown to aggregate, a much larger calcium response was shown to be mediated through the TRPV4 receptor; as TRPV4 receptor knockdown (but not AMYR inhibition) decreased the rise in intracellular calcium [83].
RAMP1 was detected in the hippocampal neurons studied via immunohistochemistry, inspiring writers to suggest that RAMP1 is the prominent receptor component responsible for this function [83]. This, however, is not in agreement with previous findings indicating that AMYR3 overexpressing COS7 cells exhibited 2-fold greater calcium mobilization than COS7 cells over-expressing AMYR1 [80]. The same study also evaluated calcium mobilization in HEK cells overexpressing either AMYR1 or AMYR3 and found no significant difference between the two [80]. Due to these inconclusive results, future studies investigating the particular AMYR responsible for recruitment of the TRPV4 receptor in primary hippocampal neurons and its relevance to cognition is warranted.
The findings of Zhang et al. regarding the role of TRPV4 in amylin signaling are supported by prior findings indicating hAmy’s ability to induce increases in intracellular calcium in murine pancreatic ß-cells [82]. TRP channel inhibition and TRPV4 receptor knockdown via siRNA prevented hAmy-induced calcium increases and reduced hAmy-triggered cell death [82]. Amylin aggregates were found on the plasma membrane adjacent to abnormal invaginations, suggesting that the TRPV4 receptor may be able to sense changes to the cell membrane induced by extracellular amylin aggregates [82].
Excitotoxicity; the result of excessive glutamate release, and subsequent NMDA and AMPA receptor activation increase intracellular calcium levels. While low doses of calcium govern a wide array of cellular processes, too much calcium can overwhelm calcium regulatory mechanisms and eventually cause cell death [116]. Aberrant calcium influx plays a role in neuronal dysfunction, inflammation, mitochondrial dysfunction, OS and apoptosis, all of which are closely associated with AD and T2D [117–119]. As hAmy and Aß have also been shown to play a role in each of the pathological features mentioned above, it is possible that their toxicity is mediated through the TRPV4 receptor via aberrant calcium influx and excitotoxicity. This theory is supported by the detection of hAmy oligomers and plaques in the high hAmy dose solution; but not the low hAmy dose solution, suggesting that the AMYR and TRPV4 receptors mediate some of the cellular dysfunction and AD-like pathological development driven by toxic amyloid signaling. It is likely that Aß may activate a similar calcium signaling cascade, but these experiments have not been done.
5. INFLAMMATION
As mentioned previously, amylin has been shown to aggregate, forming oligomers and plaques when it reaches higher concentrations, such as those observed in T2D. Amylin aggregates have been shown to induce inflammation in the pancreatic islets, contributing to T2D pathogenesis [120]. Amylin, along with Aß, is also thought to play a modulating role in inflammation associated with Alzheimer’s Disease (AD) [121, 122].
Amylin oligomers have been consistently shown to exert a proinflammatory effect. Human amylin (hAmy) aggregates stimulated secretion of numerous proinflammatory cytokines from mouse bone marrow-derived macrophages [120]. Rats overexpressing hAmy in the pancreas exhibited elevated levels of proinflammatory cytokines TNF-α and IL-6 in brain homogenates, along with a simultaneous downregulation in anti-inflammatory cytokine, IL-10, when compared to wild type rats [98]. Importantly, this increase in inflammatory response corresponded with an increase in oligomerized amylin brain levels. Amylin oligomers have also been shown to trigger the NLRP3 inflammasome, which is known to trigger immune responses, and generate mature IL-1ß in vitro [123]. The activation of the NLRP3 inflammasome is mediated via CD36, which plays a role in the conversion of prefibrillar hAmy and Aß into amyloids, leading to lysosomal disruption, activation of the NLRP3 inflammasome and IL-1ß production [124]. Aß and amylin fibril administration to murine microglia and THP-1 human monocytes increased IL-1ß, tumor necrosis factor-alpha (TNFα), IL-6, IL-8, and macrophage inflammatory protein1-α and 1-ß secretion [125].
It is important to note that rat amylin has produced no proinflammatory effect where hAmy aggregates did [98, 120, 124, 125]. Similarly, PRAM exerted anti-inflammatory effects by way of decreased hippocampal expression of the inflammatory marker COX-2 when administered peripherally to SAMP8 mice [107].
The role that monomeric hAmy plays in inflammation modulation, however, is less clear as there is a discrepancy in results. Peripheral administration of hAmy to 5xFAD mice has been shown to rescue changes in Cd68 genetic expression along with a module of genes related to inflammation, bringing expression levels back to normal [121]. Peripheral hAmy treatment was also shown to decrease Iba1 in the cortex, thalamus and the hippocampus, and decreased CD68 in the cortex; all of which were attenuated by AC253 administration [110]. Treatment of both hAmy and CGRP was shown to be effective against mouse ear oedema induced by croton oil and acetic acid-induced peritonitis [130]. As this effect was shown to be blocked by CGRP [8–37], which has a higher affinity to the CGRP receptor than the AMYR, it is possible that this effect was mediated more through the CGRP receptor than the AMYR.
On the other hand, studies have suggested a pro-inflammatory response induced by amylin. Plasma amylin levels were shown to positively correlate with C-reactive protein (CRP) and IL-6 inflammatory markers in healthy subjects [126]. Amylin and CGRP were shown to be upregulated in lumbar dorsal root ganglia following adjuvant-induced inflammation, suggesting a pro-inflammatory response [127]. Treatment of monomeric hAmy, along with oligomeric Aß, induced activation of the NLRP3 inflammasome and increased subsequent release of cytokines TNFα and IL-1ß, as well as caspase-1 in human fetal microglia (HFMs) & BV2 cells, all of which was diminished by AC253 administration [124]. The study also indicated that five weeks of peripheral AC253 administration to 5xFAD mice decreased brain levels of Iba-1, CD68, NLRP3, caspase-1, TNFα and IL-1ß [122]. AC253 administration was also shown to decrease Iba1 brain levels in TgCRND8 mice. These findings highlight the discrepancy in the field regarding the beneficial effects reported from both AMYR activation as well as AMYR inhibition, as discussed above and further reviewed in Grizzanti et al. [37].
It is highly possible that the discrepancy in results regarding monomeric hAmy is due to the concentration of amylin administered. Sources have reported that amylin, as well as Aß, exert a proinflammatory response at concentrations greater than 4uM [128]. It is difficult to compare and specify molarity in the previously mentioned in vivo studies as several pharmacological factors impact drug administration, uptake, half-life and signaling. It is also possible that these discrepancies are products of a more complex relationship between amylin signaling and the observed therapeutic effects that have yet to be discovered.
RAMP1 appears to be necessary for anti-inflammatory effects, as RAMP1 deficient mice exhibited higher proinflammatory cytokine serum levels [129]. It is difficult to determine the role of amylin signaling in this regard, however, as CGRP signaling is known to exert anti-inflammatory effects, and the CGRP receptor is composed of the CalcR and RAMP1. Alternatively, RAMP3 knockdown via siRNA in microglial BV-2 cells abolished the amylin-mediated reduction in the inflammation marker, Cd68 [121]. This suggests that the observed decrease in 5xFAD AD mouse model cortical Cd68 in response to IP treatment of hAmy is mediated through the AMYR3 receptor [121]. Further studies are necessary to elucidate the role of specific AMYR subtypes responsible for mediating amylin’s role in inflammation.
CONCLUSION
T2D is a well-known risk factor for the development of AD, but the specific mechanism responsible for AD development remains to be determined. Both diseases share common pathological features include inflammation, mitochondrial dysfunction, OS, decreased brain metabolism, impaired metabolic hormone signaling & resistance, amyloid accumulation and cognitive decline, thus determining primary components linking the two diseases has been challenging.
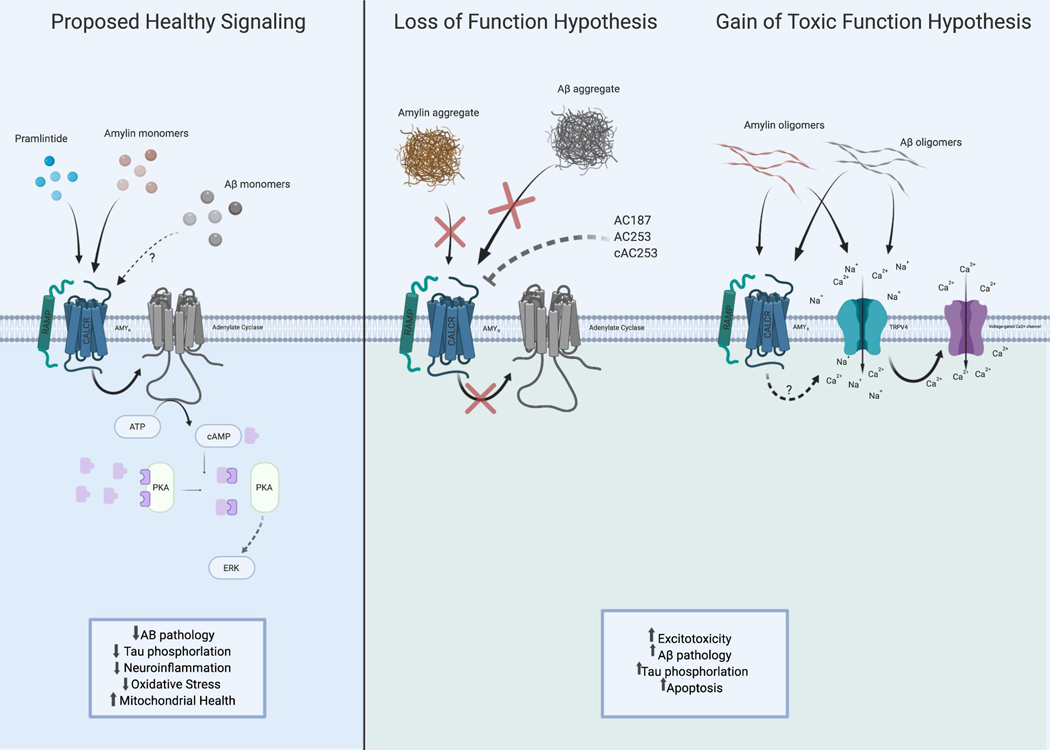
Amylin is a metabolic hormone that is affected in both diseases and that by its biochemical nature, amyloid, sits at the nexus of the relationship between these two diseases. However, as described in this review, the signaling mechanisms of this hormone, as well as evidence of both pathogenic and neuroprotective effects of amylin replacement, make deciphering the role of this hormone difficult at best. The hypothesized amylin signaling mechanisms that can lead to neuroprotective and pathogenic outcomes are summarized in Fig. 1. Amylin replacement therapy has shown effectiveness in the treatment of T2D and promise in improving function and reducing AD pathology in AD rodent models. However, whether this benefit is mediated directly through the restoration of lost amylin signaling, or indirectly by way of inhibiting AMYR-mediated Aß signaling or by way of enhancing leptin or insulin signaling remains to be clarified. Similarly, a clear dissection of which receptor complex or receptor system is involved in both pathogenic and neuroprotective effects of this hormone is necessary. This is critical for our ability to understand how amylin signaling regulated AD development and/or prevention but also to understand several other functions in which amylin is involved. Taken together, the exploration of this little-understood amyloid peptide, amylin, within the CNS in both healthy and pathological states is likely to lead to not only potential novel therapies for AD but a better understanding of other systems and functions that go beyond this devastating disease.
Fig. 1.
The proposed signaling relationships between amylin, pramlintide (PRAM), and amyloid beta (Aβ) as they exist in monomer, oligomer and aggregate states when signaling through the amylin receptor (CALCR + RAMP1–3). Left: Proposed healthy state signaling of amylin, PRAM and possibly Aβ as monomers activating AMYR in the brain leads to downstream adenylate cyclase activation to increase ERK signaling that leads to increased neuroprotective effects. Right: Loss of Function Hypothesis: amylin and Aβ aggregates (also mimicked by AMYR antagonist) may serve as a “loss of function” of normal AMYR downstream signaling during Alzheimer’s disease (AD) or metabolic dysregulation by blocking the receptor. It is proposed that due to this loss of amylin, and possibly Aβ, there will be toxic consequences such as increased Aβ pathology, Tau phosphorylation and apoptosis. Gain of Toxic Function Hypothesis: Higher concentrations or amylin/ Aβ oligomers activating AMYR may cause the recruitment of another receptor, TRPV4, in state of disease or pathology such as AD or Type II Diabetes. This activation of TRPV4, a non-selective cation channel, allows cation influx which then further induces voltage-gated Ca2+ ion channels to open leading to an excitotoxicity state due to chronic intracellular Ca2+. Created with BioRender.com.
FUNDING
Funding for this article was provided by the National Institutes of Aging grant 1R15AG050292-01A1.
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Alzheimer’s Association Report 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 2018; 14(3): 367–429. 10.1016/j.jalz.2018.02.001 [DOI] [Google Scholar]
- [2].Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, van der Flier WM. Alzheimer’s disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimers Dement 2015; 11(11): 1349–57. 10.1016/j.jalz.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers 2015; 1: 15056. 10.1038/nrdp.2015.56 [DOI] [PubMed] [Google Scholar]
- [4].Wu L, Rosa-Neto P, Hsiung GY, et al. Early-onset familial Alzheimer’s disease (EOFAD). The Canadian J Neurol Sci 2012; 39(4): 436–5. [DOI] [PubMed] [Google Scholar]
- [5].van der Flier WM, Pijnenburg YA, Fox NC, Scheltens P. Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE ɛ4 allele. Lancet Neurol 2011; 10(3): 280–8. 10.1016/S1474-4422(10)70306-9 [DOI] [PubMed] [Google Scholar]
- [6].Golde TE, Eckman CB, Younkin SG. Biochemical detection of Abeta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochim Biophys Acta 2000; 1502(1): 172–87. 10.1016/S0925-4439(00)00043-0 [DOI] [PubMed] [Google Scholar]
- [7].LaFerla FM, Oddo S. Alzheimer’s disease: Abeta, tau and synaptic dysfunction. Trends Mol Med 2005; 11(4): 170–6. 10.1016/j.molmed.2005.02.009 [DOI] [PubMed] [Google Scholar]
- [8].Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer’s disease. Biochim Biophys Acta 2000; 1502(1): 139–44. 10.1016/S0925-4439(00)00040-5 [DOI] [PubMed] [Google Scholar]
- [9].Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015; 14(4): 388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect 1991; 3(1): 1–14. 10.1007/BF02251132 [DOI] [PubMed] [Google Scholar]
- [11].Niikura T, Tajima H, Kita Y. Neuronal cell death in Alzheimer’s disease and a neuroprotective factor, humanin. Curr Neuropharmacol 2006; 4(2): 139–47. 10.2174/157015906776359577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 1986; 83(13): 4913–7. 10.1073/pnas.83.13.4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 2007; 8(9): 663–72. 10.1038/nrn2194 [DOI] [PubMed] [Google Scholar]
- [14].Mandelkow EM, Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol 1998; 8(11): 425–7. 10.1016/S0962-8924(98)01368-3 [DOI] [PubMed] [Google Scholar]
- [15].Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2012; 2(5): a006270. 10.1101/cshperspect.a006270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nhan HS, Chiang K, Koo EH. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol 2015; 129(1): 1–19. 10.1007/s00401-014-1347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Steinerman JR, Irizarry M, Scarmeas N, et al. Distinct pools of beta-amyloid in Alzheimer disease-affected brain: a clinicopathologic study. Arch Neurol 2008; 65(7): 906–12. 10.1001/archneur.65.7.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 1996; 2(8): 864–70. 10.1038/nm0896-864 [DOI] [PubMed] [Google Scholar]
- [19].De Strooper B, Saftig P, Craessaerts K, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 1998; 391(6665): 387–90. 10.1038/34910 [DOI] [PubMed] [Google Scholar]
- [20].Citron M, Westaway D, Xia W, et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med 1997; 3(1): 67–72. 10.1038/nm0197-67 [DOI] [PubMed] [Google Scholar]
- [21].Duff K, Eckman C, Zehr C, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature 1996; 383(6602): 710–3. 10.1038/383710a0 [DOI] [PubMed] [Google Scholar]
- [22].Tomita T, Maruyama K, Saido TC, et al. The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc Natl Acad Sci USA 1997; 94(5): 2025–30. 10.1073/pnas.94.5.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 2007; 8(2): 101–12. 10.1038/nrm2101 [DOI] [PubMed] [Google Scholar]
- [24].Cline EN, Bicca MA, Viola KL, Klein WL. The Amyloid-β Oligomer Hypothesis: beginning of the third decade. J Alzheimers Dis 2018; 64(s1): S567–610. 10.3233/JAD-179941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Viola KL, Klein WL. Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol 2015; 129(2): 183–206. 10.1007/s00401-015-1386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de la Monte SM. Type 3 diabetes is sporadic Alzheimers disease: mini-review. European Neuropsychopharm 2014; 24(12): 1954.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stozická Z, Zilka N, Novák M. Risk and protective factors for sporadic Alzheimer’s disease. Acta Virol 2007; 51(4): 205–22. [PubMed] [Google Scholar]
- [28].Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012; 42(5): 484–91. 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- [29].Garcia-Lara JM, Aguilar-Navarro S, Gutierrez-Robledo LM, Avila-Funes JA. The metabolic syndrome, diabetes, and Alzheimer’s disease. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion 2010; 62(4): 343–9. [PubMed] [Google Scholar]
- [30].Folch J, Pedrós I, Patraca I, Martínez N, Sureda F, Camins A. Metabolic basis of sporadic Alzeimer’s disease. Role of hormones related to energy metabolism. Curr Pharm Des 2013; 19(38): 6739–48. 10.2174/13816128113199990612 [DOI] [PubMed] [Google Scholar]
- [31].Leibson CL, Rocca WA, Hanson VA, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol 1997; 145(4): 301–8. 10.1093/oxfordjournals.aje.a009106 [DOI] [PubMed] [Google Scholar]
- [32].Kandimalla R, Thirumala V, Reddy PH. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis 2017; 1863(5): 1078–89. 10.1016/j.bbadis.2016.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 2016; 37(3): 278–316. 10.1210/er.2015-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Palermo A, Maggi D, Maurizi AR, Pozzilli P, Buzzetti R. Prevention of type 2 diabetes mellitus: is it feasible? Diabetes Metab Res Rev 2014; 30(Suppl. 1): 4–12. 10.1002/dmrr.2513 [DOI] [PubMed] [Google Scholar]
- [35].DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 1992; 15(3): 318–68. 10.2337/diacare.15.3.318 [DOI] [PubMed] [Google Scholar]
- [36].U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44(11): 1249–58. 10.2337/diab.44.11.1249 [DOI] [PubMed] [Google Scholar]
- [37].Grizzanti J, Corrigan R, Servizi S, Casadesus G. Amylin signaling in diabetes and alzheimer’s disease: therapy or pathology? J Neurol Neuromedicine 2019; 4(1):12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol 2004; 26(8): 1044–80. 10.1080/13803390490514875 [DOI] [PubMed] [Google Scholar]
- [39].Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D Jr. Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides 1990; 11(3): 467–72. 10.1016/0196-9781(90)90044-6 [DOI] [PubMed] [Google Scholar]
- [40].Wallum BJ, Taborsky GJ Jr, Porte D Jr, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab 1987; 64(1): 190–4. 10.1210/jcem-64-1-190 [DOI] [PubMed] [Google Scholar]
- [41].Gil-Bea FJ, Solas M, Solomon A, et al. Insulin levels are decreased in the cerebrospinal fluid of women with prodomal Alzheimer’s disease. J Alzheimers Dis 2010; 22(2): 405–13. 10.3233/JAD-2010-100795 [DOI] [PubMed] [Google Scholar]
- [42].Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol Neurobiol 2013; 47(1): 145–71. 10.1007/s12035-012-8339-9 [DOI] [PubMed] [Google Scholar]
- [43].Berent S, Giordani B, Foster N, et al. Neuropsychological function and cerebral glucose utilization in isolated memory impairment and Alzheimer’s disease. J Psychiatr Res 1999; 33(1): 7–16. 10.1016/S0022-3956(98)90048-6 [DOI] [PubMed] [Google Scholar]
- [44].Willette AA, Bendlin BB, Starks EJ, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol 2015; 72(9): 1013–20. 10.1001/jamaneurol.2015.0613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opinion Investig Drugs (London, England : 2000) 2009; 10(10): 1049–60. [PMC free article] [PubMed] [Google Scholar]
- [46].de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis 2005; 7(1): 45–61. 10.3233/JAD-2005-7106 [DOI] [PubMed] [Google Scholar]
- [47].Grillo CA, Piroli GG, Lawrence RC, et al. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes 2015; 64(11): 3927–36. 10.2337/db15-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA 2001; 98(6): 3561–6. 10.1073/pnas.051634698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol 2001; 177(1–2): 125–34. 10.1016/S0303-7207(01)00455-5 [DOI] [PubMed] [Google Scholar]
- [50].Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs 2003; 17(1): 27–45. 10.2165/00023210-200317010-00003 [DOI] [PubMed] [Google Scholar]
- [51].Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology 2004; 63(7): 1187–92. 10.1212/01.WNL.0000140292.04932.87 [DOI] [PubMed] [Google Scholar]
- [52].Medina M, Garrido JJ, Wandosell FG. Modulation of GSK-3 as a therapeutic strategy on tau pathologies. Front Mol Neurosci 2011; 4: 24. 10.3389/fnmol.2011.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhao WQ, Lacor PN, Chen H, et al. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric abeta. J Biol Chem 2009; 284(28): 18742–53. 10.1074/jbc.M109.011015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pearson-Leary J, McNay EC. Intrahippocampal administration of amyloid-β(1–42) oligomers acutely impairs spatial working memory, insulin signaling, and hippocampal metabolism. J Alzheimers Dis 2012; 30(2): 413–22. 10.3233/JAD-2012-112192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang Y, Zhou B, Zhang F, et al. Amyloid-β induces hepatic insulin resistance by activating JAK2/STAT3/SOCS-1 signaling pathway. Diabetes 2012; 61(6): 1434–43. 10.2337/db11-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shiiki T, Ohtsuki S, Kurihara A, et al. Brain insulin impairs amyloid-β(1–40) clearance from the brain. J Neurosci 2004; 24(43): 9632–7. 10.1523/JNEUROSCI.2236-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Messier C, Teutenberg K. The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer’s disease. Neural Plast 2005; 12(4): 311–28. 10.1155/NP.2005.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Qiu WQ, Walsh DM, Ye Z, et al. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J Biol Chem 1998; 273(49): 32730–8. 10.1074/jbc.273.49.32730 [DOI] [PubMed] [Google Scholar]
- [59].Bennett RG, Hamel FG, Duckworth WC. An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures. Diabetes 2003; 52(9): 2315–20. 10.2337/diabetes.52.9.2315 [DOI] [PubMed] [Google Scholar]
- [60].Hwang JJ, Chan JL, Ntali G, Malkova D, Mantzoros CS. Leptin does not directly regulate the pancreatic hormones amylin and pancreatic polypeptide: interventional studies in humans. Diabetes Care 2008; 31(5): 945–51. 10.2337/dc07-2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev 2015; 67(3): 564–600. 10.1124/pr.115.010629 [DOI] [PubMed] [Google Scholar]
- [62].Christopoulos G, Perry KJ, Morfis M, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol 1999; 56(1): 235–42. 10.1124/mol.56.1.235 [DOI] [PubMed] [Google Scholar]
- [63].Flahaut M, Rossier BC, Firsov D. Respective roles of calcitonin receptor-like receptor (CRLR) and receptor activity-modifying proteins (RAMP) in cell surface expression of CRLR/RAMP heterodimeric receptors. J Biol Chem 2002; 277(17): 14731–7. 10.1074/jbc.M112084200 [DOI] [PubMed] [Google Scholar]
- [64].Gorn AH, Lin HY, Yamin M, et al. Cloning, characterization, and expression of a human calcitonin receptor from an ovarian carcinoma cell line. J Clin Invest 1992; 90(5): 1726–35. 10.1172/JCI116046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Masi L, Brandi ML. Calcitonin and calcitonin receptors. Clin Cases Miner Bone Metab 2007; 4(2): 117–22. [PMC free article] [PubMed] [Google Scholar]
- [66].Becskei C, Riediger T, Zünd D, Wookey P, Lutz TA. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res 2004; 1030(2): 221–33. 10.1016/j.brainres.2004.10.012 [DOI] [PubMed] [Google Scholar]
- [67].Bower RL, Eftekhari S, Waldvogel HJ, et al. Mapping the calcitonin receptor in human brain stem. Am J Physiol Regul Integr Comp Physiol 2016; 310(9): R788–93. 10.1152/ajpregu.00539.2015 [DOI] [PubMed] [Google Scholar]
- [68].Sexton PM, Findlay DM, Martin TJ. Calcitonin. Curr Med Chem 1999; 6(11): 1067–93. [PubMed] [Google Scholar]
- [69].Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 2002; 54(2): 233–46. 10.1124/pr.54.2.233 [DOI] [PubMed] [Google Scholar]
- [70].Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther 2000; 294(1): 61–72. [PubMed] [Google Scholar]
- [71].Fraser NJ, Wise A, Brown J, McLatchie LM, Main MJ, Foord SM. The amino terminus of receptor activity modifying proteins is a critical determinant of glycosylation state and ligand binding of calcitonin receptor-like receptor. Mol Pharmacol 1999; 55(6): 1054–9. 10.1124/mol.55.6.1054 [DOI] [PubMed] [Google Scholar]
- [72].Sexton PM, Albiston A, Morfis M, Tilakaratne N. Receptor activity modifying proteins. Cell Signal 2001; 13(2): 73–83. 10.1016/S0898-6568(00)00143-1 [DOI] [PubMed] [Google Scholar]
- [73].Ueda T, Ugawa S, Saishin Y, Shimada S. Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Res Mol Brain Res 2001; 93(1): 36–45. 10.1016/S0169-328X(01)00179-6 [DOI] [PubMed] [Google Scholar]
- [74].Jhamandas JH, Li Z, Westaway D, Yang J, Jassar S, MacTavish D. Actions of β-amyloid protein on human neurons are expressed through the amylin receptor. Am J Pathol 2011; 178(1): 140–9. 10.1016/j.ajpath.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mietlicki-Baase EG, Rupprecht LE, Olivos DR, et al. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology 2013; 38(9): 1685–97. 10.1038/npp.2013.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Stachniak TJ, Krukoff TL. Receptor activity modifying protein 2 distribution in the rat central nervous system and regulation by changes in blood pressure. J Neuroendocrinol 2003; 15(9): 840–50. 10.1046/j.1365-2826.2003.01064.x [DOI] [PubMed] [Google Scholar]
- [77].J Gingell J, Simms J, Barwell J, et al. An allosteric role for receptor activity-modifying proteins in defining GPCR pharmacology. Cell Discov 2016; 2: 16012. 10.1038/celldisc.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Muff R, Bühlmann N, Fischer JA, Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or −3. Endocrinology 1999; 140(6): 2924–7. 10.1210/endo.140.6.6930 [DOI] [PubMed] [Google Scholar]
- [79].Lee S-M, Hay DL, Pioszak AA. Calcitonin and amylin receptor peptide interaction mechanisms: insights into peptide-binding modes and allosteric modulation of the calcitonin receptor by receptor activity-modifying proteins. J Biol Chem 2016; 291(16): 8686–700. 10.1074/jbc.M115.713628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Morfis M, Tilakaratne N, Furness SG, et al. Receptor activity-modifying proteins differentially modulate the G protein-coupling efficiency of amylin receptors. Endocrinology 2008; 149(11): 5423–31. 10.1210/en.2007-1735 [DOI] [PubMed] [Google Scholar]
- [81].Woolley MJ, Conner AC. Comparing the molecular pharmacology of CGRP and adrenomedullin. Curr Protein Pept Sci 2013; 14(5): 358–74. 10.2174/13892037113149990053 [DOI] [PubMed] [Google Scholar]
- [82].Casas S, Novials A, Reimann F, Gomis R, Gribble FM. Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia 2008; 51(12): 2252–62. 10.1007/s00125-008-1111-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhang N, Yang S, Wang C, et al. Multiple target of hAmylin on rat primary hippocampal neurons. Neuropharmacology 2017; 113(Pt A): 241–51. 10.1016/j.neuropharm.2016.07.008 [DOI] [PubMed] [Google Scholar]
- [84].Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci 2009; 32(4): 215–24. 10.1016/j.tins.2008.12.006 [DOI] [PubMed] [Google Scholar]
- [85].Bhogal R, Smith DM, Bloom SR. Investigation and characterization of binding sites for islet amyloid polypeptide in rat membranes. Endocrinology 1992; 130(2): 906–13. [DOI] [PubMed] [Google Scholar]
- [86].Sexton PM, Paxinos G, Kenney MA, Wookey PJ, Beaumont K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience 1994; 62(2): 553–67. 10.1016/0306-4522(94)90388-3 [DOI] [PubMed] [Google Scholar]
- [87].Soudy R, Patel A, Fu W, Kaur K, et al. Cyclic AC253, a novel amylin receptor antagonist, improves cognitive deficits in a mouse model of Alzheimer’s disease. Alzheimer’s & dementia (New York, N Y) 2017; 3(1): 44–56. 10.1016/j.trci.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kimura R, MacTavish D, Yang J, Westaway D, Jhamandas JH. Pramlintide Antagonizes beta amyloid (Aβ)- and human amylin-induced depression of hippocampal long-term potentiation. Mol Neurobiol 2017; 54(1): 748–54. 10.1007/s12035-016-9684-x [DOI] [PubMed] [Google Scholar]
- [89].Kimura R, MacTavish D, Yang J, Westaway D, Jhamandas JH. Beta amyloid-induced depression of hippocampal long-term potentiation is mediated through the amylin receptor. J Neurosci 2012; 32(48): 17401–6. 10.1523/JNEUROSCI.3028-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Szabó ÉR, Cservenák M, Dobolyi A. Amylin is a novel neuropeptide with potential maternal functions in the rat. FASEB J 2012; 26(1): 272–81. 10.1096/fj.11-191841 [DOI] [PubMed] [Google Scholar]
- [91].Dobolyi A. Central amylin expression and its induction in rat dams. J Neurochem 2009; 111(6): 1490–500. 10.1111/j.1471-4159.2009.06422.x [DOI] [PubMed] [Google Scholar]
- [92].D’Este L, Casini A, Wimalawansa SJ, Renda TG. Immunohistochemical localization of amylin in rat brainstem. Peptides 2000; 21(11): 1743–9. 10.1016/S0196-9781(00)00325-9 [DOI] [PubMed] [Google Scholar]
- [93].D’Este L, Wimalawansa SJ, Renda TG. Distribution of amylin-immunoreactive neurons in the monkey hypothalamus and their relationships with the histaminergic system. Arch Histol Cytol 2001; 64(3): 295–303. 10.1679/aohc.64.295 [DOI] [PubMed] [Google Scholar]
- [94].Jaikaran ET, Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim Biophys Acta 2001; 1537(3): 179–203. 10.1016/S0925-4439(01)00078-3 [DOI] [PubMed] [Google Scholar]
- [95].Hull RL, Andrikopoulos S, Verchere CB, et al. Increased dietary fat promotes islet amyloid formation and β-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes 2003; 52(2): 372–9. 10.2337/diabetes.52.2.372 [DOI] [PubMed] [Google Scholar]
- [96].Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia 1978; 15(5): 417–21. 10.1007/BF01219652 [DOI] [PubMed] [Google Scholar]
- [97].Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 1998; 19(5): 883–9. 10.1016/S0196-9781(98)00018-7 [DOI] [PubMed] [Google Scholar]
- [98].Srodulski S, Sharma S, Bachstetter AB, et al. Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener 2014; 9(1): 30. 10.1186/1750-1326-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Verma N, Ly H, Liu M, et al. Intraneuronal amylin deposition, peroxidative membrane injury and increased il-1β synthesis in brains of alzheimer’s disease patients with type-2 diabetes and in diabetic HIP rats. J Alzheimers Dis 2016; 53(1): 259–72. 10.3233/JAD-160047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Qiu WQ, Zhu H. Amylin and its analogs: a friend or foe for the treatment of Alzheimer’s disease? Front Aging Neurosci 2014; 6: 186. 10.3389/fnagi.2014.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 2014; 6(4): 37. 10.1186/alzrt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hyde C, Peters J, Bond M, et al. Evolution of the evidence on the effectiveness and cost-effectiveness of acetylcholinesterase inhibitors and memantine for Alzheimer’s disease: systematic review and economic model. Age Ageing 2013; 42(1): 14–20. 10.1093/ageing/afs165 [DOI] [PubMed] [Google Scholar]
- [103].Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA 1990; 87(13): 5036–40. 10.1073/pnas.87.13.5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang H, Ridgway Z, Cao P, Ruzsicska B, Raleigh DP. Analysis of the ability of pramlintide to inhibit amyloid formation by human islet amyloid polypeptide reveals a balance between optimal recognition and reduced amyloidogenicity. Biochemistry 2015; 54(44): 6704–11. 10.1021/acs.biochem.5b00567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Qiu WQ, Au R, Zhu H, et al. Positive association between plasma amylin and cognition in a homebound elderly population. J Alzheimers Dis 2014; 42(2): 555–63. 10.3233/JAD-140210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Qiu WQ, Wallack M, Dean M, Liebson E, Mwamburi M, Zhu H. Association between amylin and amyloid-β peptides in plasma in the context of apolipoprotein E4 allele. PLoS One 2014; 9(2): e88063. 10.1371/journal.pone.0088063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Adler BL, Yarchoan M, Hwang HM, et al. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol Aging 2014; 35(4): 793–801. 10.1016/j.neurobiolaging.2013.10.076 [DOI] [PubMed] [Google Scholar]
- [108].Patrick S, Corrigan R, Grizzani J, et al. Neuroprotective effects of the amylin analog, pramlintide, on alzheimer’s disease are associated with oxidative stress regulation mechanisms. J Alzheimer’s Disease 2019; (Preprint): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhu H, Wang X, Wallack M, et al. Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer’s disease. Mol Psychiatry 2015; 20(2): 252–62. 10.1038/mp.2014.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhu H, Xue X, Wang E, et al. Amylin receptor ligands reduce the pathological cascade of Alzheimer’s disease. Neuropharmacology 2017; 119: 170–81. 10.1016/j.neuropharm.2017.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lim Y-A, Ittner LM, Lim YL, Götz J. Human but not rat amylin shares neurotoxic properties with Abeta42 in long-term hippocampal and cortical cultures. FEBS Lett 2008; 582(15): 2188–94. 10.1016/j.febslet.2008.05.006 [DOI] [PubMed] [Google Scholar]
- [112].Jhamandas JH, MacTavish D. Antagonist of the amylin receptor blocks beta-amyloid toxicity in rat cholinergic basal forebrain neurons. J Neurosci 2004; 24(24): 5579–84. 10.1523/JNEUROSCI.1051-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Fu W, Ruangkittisakul A, MacTavish D, Shi JY, Ballanyi K, Jhamandas JH. Amyloid β (Aβ) peptide directly activates amylin-3 receptor subtype by triggering multiple intracellular signaling pathways. J Biol Chem 2012; 287(22): 18820–30. 10.1074/jbc.M111.331181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Jhamandas JH, Mactavish D. beta-Amyloid protein (Abeta) and human amylin regulation of apoptotic genes occurs through the amylin receptor. Apoptosis 2012; 17(1): 37–47. [DOI] [PubMed] [Google Scholar]
- [115].Gingell JJ, Burns ER, Hay DL. Activity of pramlintide, rat and human amylin but not Aβ1–42 at human amylin receptors. Endocrinology 2014; 155(1): 21–6. 10.1210/en.2013-1658 [DOI] [PubMed] [Google Scholar]
- [116].Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003; 34(4–5): 325–37. 10.1016/S0143-4160(03)00141-6 [DOI] [PubMed] [Google Scholar]
- [117].Sama DM, Norris CM. Calcium dysregulation and neuroinflammation: discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res Rev 2013; 12(4): 982–95. 10.1016/j.arr.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Magi S, Castaldo P, Macrì ML, et al. Intracellular calcium dysregulation: implications for Alzheimer’s disease. BioMed Res Int 2016; 2016: 6701324. 10.1155/2016/6701324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Arruda AP, Hotamisligil GS. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab 2015; 22(3): 381–97. 10.1016/j.cmet.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Westwell-Roper C, Dai DL, Soukhatcheva G, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol 2011; 187(5): 2755–65. 10.4049/jimmunol.1002854 [DOI] [PubMed] [Google Scholar]
- [121].Wang E, Zhu H, Wang X, et al. Amylin treatment reduces neuroinflammation and ameliorates abnormal patterns of gene expression in the cerebral cortex of an Alzheimer’s disease mouse model. J Alzheimers Dis 2017; 56(1): 47–61. 10.3233/JAD-160677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Fu W, Vukojevic V, Patel A, et al. Role of microglial amylin receptors in mediating beta amyloid (Aβ)-induced inflammation. J Neuroinflammation 2017; 14(1): 199. 10.1186/s12974-017-0972-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 2010; 11(10): 897–904. 10.1038/ni.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 2013; 14(8): 812–20. 10.1038/ni.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Yates SL, Burgess LH, Kocsis-Angle J, et al. Amyloid β and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J Neurochem 2000; 74(3): 1017–25. 10.1046/j.1471-4159.2000.0741017.x [DOI] [PubMed] [Google Scholar]
- [126].Hou X, Sun L, Li Z, et al. Associations of amylin with inflammatory markers and metabolic syndrome in apparently healthy Chinese. PLoS One 2011; 6(9): e24815. 10.1371/journal.pone.0024815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mulder H, Zhang Y, Danielsen N, Sundler F. Islet amyloid polypeptide and calcitonin gene-related peptide expression are downregulated in dorsal root ganglia upon sciatic nerve transection. Brain Res Mol Brain Res 1997; 47(1–2): 322–30. 10.1016/S0169-328X(97)00060-0 [DOI] [PubMed] [Google Scholar]
- [128].Gitter BD, Cox LM, Carlson CD, May PC. Human amylin stimulates inflammatory cytokine secretion from human glioma cells. Neuroimmunomodulation 2000; 7(3): 147–52. 10.1159/000026432 [DOI] [PubMed] [Google Scholar]
- [129].Tsujikawa K, Yayama K, Hayashi T, et al. Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc Natl Acad Sci USA 2007; 104(42): 16702–7. 10.1073/pnas.0705974104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Clementi G, Busa L, de Bernardis E, Prato A, Drago F. Effects of centrally injected amylin on sexually behavior of male rats. Peptides 1999; 20(3): 379–82. 10.1016/S0196-9781(98)00166-1 [DOI] [PubMed] [Google Scholar]