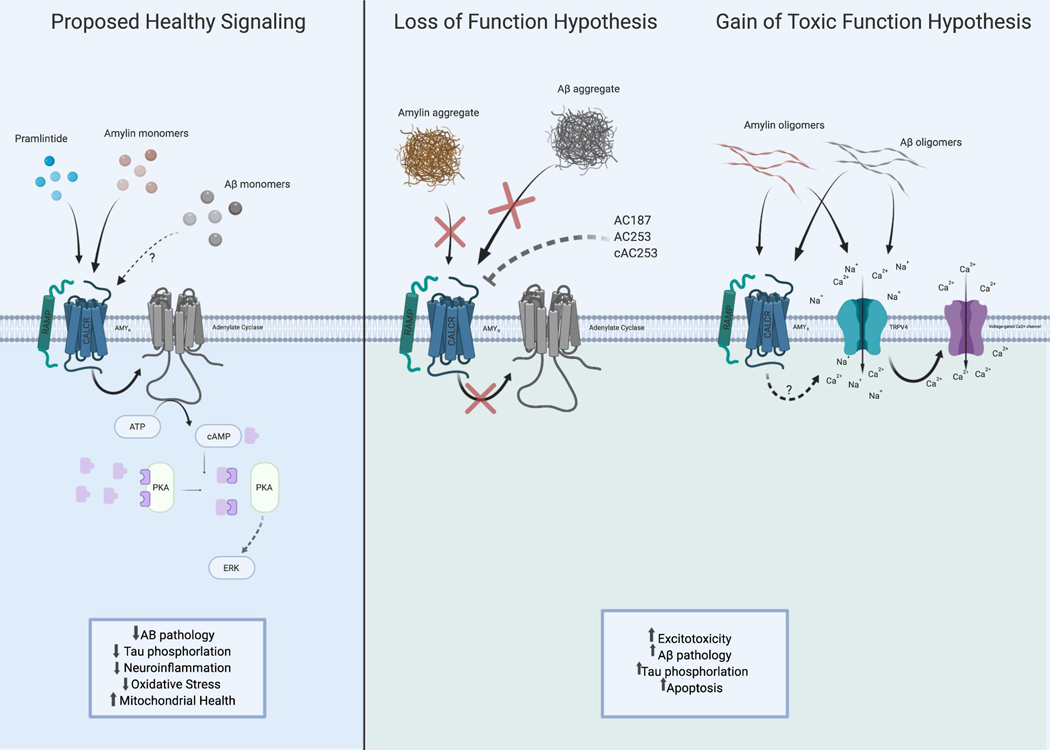
Fig. 1.
The proposed signaling relationships between amylin, pramlintide (PRAM), and amyloid beta (Aβ) as they exist in monomer, oligomer and aggregate states when signaling through the amylin receptor (CALCR + RAMP1–3). Left: Proposed healthy state signaling of amylin, PRAM and possibly Aβ as monomers activating AMYR in the brain leads to downstream adenylate cyclase activation to increase ERK signaling that leads to increased neuroprotective effects. Right: Loss of Function Hypothesis: amylin and Aβ aggregates (also mimicked by AMYR antagonist) may serve as a “loss of function” of normal AMYR downstream signaling during Alzheimer’s disease (AD) or metabolic dysregulation by blocking the receptor. It is proposed that due to this loss of amylin, and possibly Aβ, there will be toxic consequences such as increased Aβ pathology, Tau phosphorylation and apoptosis. Gain of Toxic Function Hypothesis: Higher concentrations or amylin/ Aβ oligomers activating AMYR may cause the recruitment of another receptor, TRPV4, in state of disease or pathology such as AD or Type II Diabetes. This activation of TRPV4, a non-selective cation channel, allows cation influx which then further induces voltage-gated Ca2+ ion channels to open leading to an excitotoxicity state due to chronic intracellular Ca2+. Created with BioRender.com.