Abstract
In this review, we present a discussion of diabetes and its complications, including the macrovascular and microvascular effects, with the latter of consequence to the retina. We will discuss the anatomy and physiology of the retina, including aspects of metabolism and mechanisms of oxygenation, with the latter accomplished via a combination of the retinal and choroidal blood circulations. Both of these vasculatures are altered in diabetes, with the retinal circulation intimately involved in the pathology of diabetic retinopathy. The later stages of diabetic retinopathy involve poorly controlled angiogenesis that is of great concern, but in our discussion, we will focus more on several alterations in the retinal circulation occurring earlier in the progression of disease, including reductions in blood flow and a possible redistribution of perfusion that may leave some areas of the retina ischemic and hypoxic. Finally, we include in this review a more recent area of investigation regarding the diabetic retinal vasculature, that is, the alterations to the endothelial surface layer that normally plays a vital role in maintaining physiological functions.
Keywords: diabetic retinopathy, retinal blood flow, endothelial surface layer, platelet endothelial cell adhesion molecule, glycocalyx
Introduction
The retinal vasculature, as with most circulatory beds, has unique physiological characteristics appropriate to the function of the tissue. For the retina, this includes delivering sufficient oxygen and nutrients to cells having a high metabolic rate, but without the vasculature being so dense as to prevent light transmission. Proper vision is threatened by any interruption or significant decrease of retinal blood flow, with these disturbances occurring early in the progression of diabetes. Determining the causes of the diabetes-induced decreases in retinal blood flow is of significant clinical concern, but have yet to be fully elucidated.
The endothelium comprises the inner lining of the vasculature, and in turn, the inner surface layer of the endothelium (molecules on the cell membrane) serves as an intermediary between the blood and endothelial function. On the surface of the endothelium are junctional molecules, adhesion molecules, and a dense layer of proteoglycans and glycosaminoglycans called the glycocalyx. The endothelial surface layer of the retinal circulation is significantly altered in diabetes, with the consequences of these changes likely to participate in the progression of retinopathy, which is a relatively recent topic of investigation in the field that will be discussed in this review.
Herein we discuss some general topics of diabetes and its complications; the anatomy and physiology of the retina; the organization and function of the posterior circulations of the eye; mechanisms that contribute to diabetic retinopathy; and diabetes-induced changes in retinal blood flow and the endothelial surface layer. Abbreviations used in the review are listed in Table 1.
TABLE 1.
LIST OF ABBREVIATIONS
| 20-HETE | 20-hydroxyeicosatetraeonic acid |
| ACE | angiotensin converting enzyme |
| AGE | advanced glycation end product |
| Ang II | angiotensin II |
| aPKC | atypical protein kinase C |
| AR | aldose reductase |
| AT | angiogensin II receptor type |
| ATP | adenosine triphosphate |
| BBB | blood brain barrier |
| BRB | blood retinal barrier |
| CAD | coronary artery disease |
| cGMP | cyclic guanine monophosphate |
| COX-2 | cyclooxygenase-2 |
| cPKC | conventional protein kinase C |
| DAG | diacylglycerol |
| DC | dendritic cell |
| EDCF | endothelium-derived constricting factor |
| eNOS | endothelial nitric oxide synthase |
| EPO | erythropoietin |
| ET | endothelin |
| FADH2 | flavin adenine dinucleotide |
| FAM3B | family with sequence similarity 3 member B |
| GAG | glycosaminoglycan |
| GC | guanylate cyclase |
| GCL | ganglion cell layer |
| GEnC | glomerular endothelial cell |
| GK | Goto-Kakizaki |
| GLUT | glucose transporter |
| HDL | high-density lipoprotein |
| HIF | hypoxia-inducible factor |
| HUVEC | human umbilical vein endothelial cell |
| iBRB | inner blood retinal barrier |
| ICAM-1 | intercellular adhesion molecule-1 |
| IDDM | insulin-dependent diabetes mellitus |
| Ig | immunoglobulin g |
| IL-1β | interleukin-1β |
| ILM | inner limiting membrane |
| INL | inner nuclear layer |
| iNOS | inducible nitric oxide synthase |
| IOP | intraocular pressure |
| IPL | inner plexiform layer |
| JAM | junctional adhesion molecule |
| JIP1 | c-Jun N- terminal kinase interacting protein-1 |
| JNK | c-Jun N- terminal kinase |
| KKS | kallikrein-kinin system |
| LDL | low-density lipoprotein |
| L-NA | Nitro-L-arginine |
| L-NAME | NG-nitro-L-arginine methyl ester |
| L-NMMA | NG-monomethyl-L-arginine |
| L-NPA | N(ω)-propyl-L-arginine |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| MCT | mean circulation time |
| MFS | major facilitator superfamily |
| MI | myocardial infarction |
| mmHg | millimeters of mercury |
| MMP | matrix metalloproteinase |
| NAD | nicotinamide adenine dinucleotide |
| NADH | nicotinamide adenine dinucleotide plus hydrogen |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NCD | non-communicable disease |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NFL | nerve fiber layer |
| nNOS | neuronal nitric oxide synthase |
| NO | nitric oxide |
| NOD | non-obese diabetic |
| NOS | nitric oxide synthase |
| NOX | nicotinamide adenine dinucleotide phosphate oxidase |
| NPDR | non-proliferative diabetic retinopathy |
| nPKC | novel protein kinase C |
| oBRB | outer blood retinal barrier |
| OLM | outer limiting membrane |
| ONL | outer nuclear layer |
| ONOO− | peroxynitrite |
| OPL | outer plexiform layer |
| P | transmural pressure |
| PaCO2 | arterial partial pressure of carbon dioxide |
| PAD | peripheral artery disease |
| PDGF | platelet-derived growth factor |
| PDR | proliferative diabetic retinopathy |
| PECAM-1 | platelet endothelial cells adhesion molecule-1 |
| PEDF | pigment epithelium derived factor |
| PGD2 | prostaglandin D2 |
| PGE1 | prostaglandin E1 |
| PGF2α | prostaglandin F2α |
| PGI2 | prostaglandin I2 |
| PK | protein kinase |
| PMA | 12-myristate 13-acetate |
| PS | phosphatidylserine |
| PVD | posterior vitreous detachment |
| pO2 | partial pressure of oxygen |
| r | vessel radius |
| RAGE | receptor for advanced glycation end product |
| RBC | red blood cell |
| RMECS | retinal microvascular endothelial cells |
| RPE | retinal pigmented epithelium |
| ROS | reactive oxygen species |
| S1P | sphingosine-1 phosphate |
| SHP-2 | Src homology region 2 domain-containing phosphatase-2 |
| SOD | superoxide dismutase |
| STAT3 | signal transducer and activator of transcription 3 |
| STZ | streptozotocin |
| T | vascular wall tension |
| TGF | transforming growth factor |
| TLR | toll-like receptor |
| TNFα | tumor necrosis factor-α |
| TNFR | tumor necrosis factor-α receptor |
| TxA2 | thromboxane A2 |
| VE-cadherin | vascular endothelial cadherin |
| VEGF | vascular endothelial growth factor |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| VSMC | vascular smooth muscle cell |
| ZO | zonula occludens |
Diabetes and Its Complications
Incidence and epidemiology
Diabetes currently afflicts millions of individuals worldwide and is considered one of the greatest health emergencies of the 21st century. According to the Center for Disease Control and Prevention, one in 10 US adults has diabetes, with a projected growth to one in three US adults by the year 2050 (2). Diabetes is one of the four non-communicable diseases (NCDs) that are responsible for around 80% of premature deaths globally, with the three other NCDs being cancer, cardiovascular, and respiratory diseases (101). The number of people diagnosed with diabetes has grown from 108 million in 1980 to 425 million in 2017, with expected growth to 629 million by the year 2045 (91). This is an alarming trend considering the massive financial burden diabetes can have on healthcare systems and individuals with diabetes. The cost of diabetes has risen from $232 billion dollars per year in 2007 to an estimated $727 billion per year in 2017, with predicted growth to $1.7 trillion for direct and indirect costs by the year 2030 (91). In the United States, there were an estimated 30 million people with diabetes (diagnosed and undiagnosed) in 2017, with expected growth to 43 million people in the year 2045 (91), making the United States the second highest number of people with diabetes following China.
Major complications of diabetes
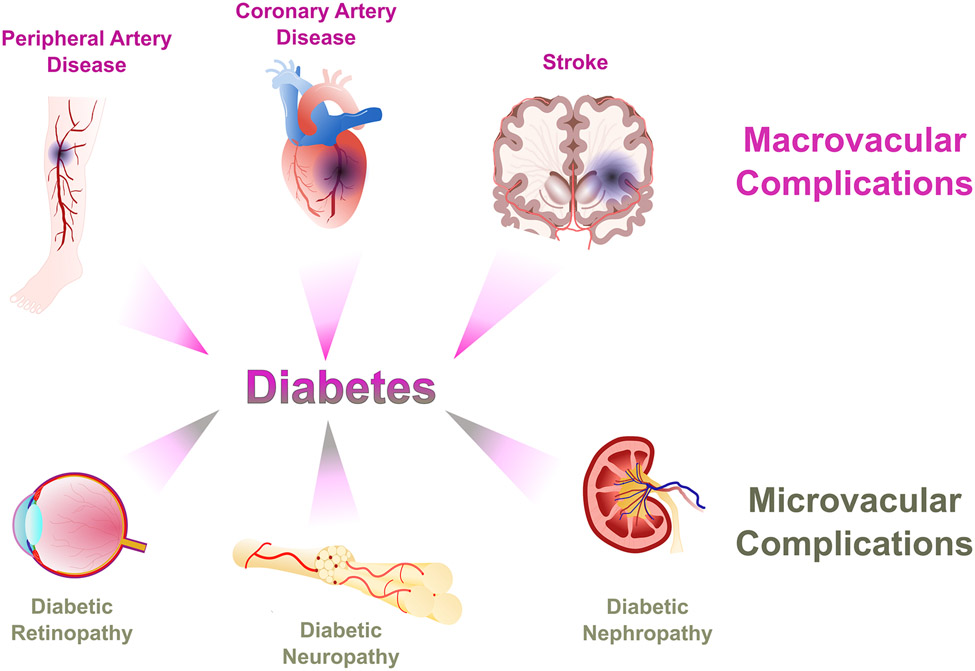
Diabetes is often associated with significant organ damage and failure, which leads to an increase in mortality rates. Almost all diabetes-related complications can be attributed to vascular damage and remodeling due to hyperglycemia. This vascular remodeling can be divided into two main categories (Figure 1) depending on the size of the affected blood vessels: 1) macrovascular complications that affect the larger blood vessels, leading to coronary artery disease (CAD), stroke, and peripheral artery disease (PAD) and 2) microvascular complications that affect the microcirculatory blood vessels, leading to diabetic retinopathy, diabetic nephropathy, and diabetic peripheral neuropathy, with the latter attributed to abnormalities within the neuronal cells resulting from microvascular dysfunction. Patients who are diagnosed with insulin-dependent diabetes mellitus (IDDM) are likely to have macrovascular or microvascular complications within 15 years of their diagnosis, with these complications occurring more in type I than type II diabetes (638).
Figure 1. Macrovascular and microvascular complications of diabetes.
Macrovascular complications include peripheral artery disease, coronary artery disease, and stroke; microvascular complications include diabetic retinopathy, neuropathy, and nephropathy.
In both macrovascular and microvascular complications, hyperglycemia is considered an independent and major risk factor that plays a vital role in the ensuing vascular damage. Under normal conditions, protective factors such as antioxidant enzymes and insulin protect the blood vessels from damage, making them less susceptible to injury (342). However, under hyperglycemic conditions, these factors are inhibited, while factors leading to vessel damage and injury are upregulated.
Blood vessels in the macrovascular system differ from those in the microvascular system with regard to structure, function, wall composition, wall thickness, and vessel diameter, yet the vascular network shares the endothelium organ system. The endothelium contains a heterogenous population of cells that have metabolic activity (described in (157)) comprised of three major energy sources: glucose, fatty acids, and amino acids. Endothelial cells arising from different vascular beds have different sizes, anatomical compartments, and express different proteins, and their response to physiological and pathological conditions can vary remarkably (14-16).
Hyperglycemia can induce endothelial cell modifications due to the disruption of homeostasis leading to damage in both the large and small blood vessels. These modifications include altered signaling, upregulation of metabolic activity, and alterations in ultrastructure (465, 524). Different molecular mechanisms can cause hyperglycemia-induced macrovascular and microvascular complications, yet both share many components that interlink with each other leading to vascular injury. An increase in vascular permeability (608), vascular cell apoptosis (206, 559), leukocyte adhesion (196), and altered blood flow (97, 239, 559) are among the shared systemic factors that contribute to both macrovascular and microvascular complications due to diabetes. Thus, the question of whether one complication precedes the other, or if they develop concurrently, is still under investigation.
Macrovascular complications of diabetes
The macrovascular system consists of large capacity blood vessels, namely the arteries and veins, in which the primary function is to rapidly deliver blood to and from the various organs of the body. Thus, any blockage or damage to these vessels can have deleterious pathological effects, which can be terminal. Insulin resistance and poor glycemic control are associated with an increased risk in the development of macrovascular diseases in diabetes (435).
One of the major contributors to macrovascular complication hallmarks in diabetes is the development of atherosclerosis in medium and large blood vessels, leading to their partial or total blockade (572), resulting in cardiovascular (coronary artery disease), cerebrovascular (stroke) and peripheral arterial diseases. Diabetes can increase the atherogenic process in these vessels. The exact mechanism is still under investigation, but hyperglycemia is considered a significant player in this process.
The development of atherosclerosis due to hyperglycemia is a complex process that involves multiple mechanisms such as the formation of advanced glycation end products (AGEs), reactive oxygen species (ROS) as a result of altered glucose metabolism, and endothelial cell dysfunction leading to altered enzymatic activity and an increase in adhesion molecule expression (481). Moreover, hyperglycemia is a major contributing factor to vascular smooth muscle cell (VSMC) proliferation in the atherosclerotic arterial intima and the formation of the atherosclerotic fibrous cap (168). Studies have indicated a possible role of proto-oncogene serine/threonine-protein kinase Pim-1 upregulation in hyperglycemia-induced VSMC proliferation and migration via the activation of signal transducer and activator of transcription 3 (STAT3)/Pim-1 signaling. Additionally, FAM3B (Family with Sequence Similarity 3 Member B), which is co-released with insulin from pancreatic β-cells upon glucose stimulation, has been found to be upregulated under hyperglycemic conditions leading to the inhibition of miR-322-5p and proliferation of VSMC (651).
Hyperglycemia can lead to metabolic disturbances and impairment in lipid metabolism (596). In type I diabetes, there are increased levels in total and low-density lipoproteins (LDLs), accompanied by lower levels in high-density lipoprotein (HDLs), with this combination leading to an increased risk of atherosclerosis development (572, 596). Furthermore, ROS and AGEs enhance atherogenesis by promoting the retention of apolipoprotein B-containing and modified LDL in the arterial intima (591). The increased expression of endothelial cell adhesion molecules such as E-selectin, P-selectin, and intercellular adhesion molecule-1 (ICAM-1) lead to adhesion of mononuclear leukocytes such as monocytes and T-cells, and their migration to the arterial intima (271, 348) where they differentiate to macrophages and dendritic cells (DCs). Monocyte-driven macrophages take up lipoprotein and differentiate to foam cells. These foam cells and other immune cells such as DCs produce cytokines and chemokines such as tumor necrosis factor-α (TNF-α) and monocyte chemoattractant protein-1 (MCP-1) that exacerbate inflammation and recruit additional immune cells (276). Moreover, the formation of the necrotic core in advanced plaques can be a result of macrophage apoptosis, which also can contribute to the release of matrix metalloproteinases (MMPs) that break down the fibrous atheroma cap, leading to atherosclerotic plaque rupture and thrombus formation (87, 395, 488). Thrombosis enhances the risk of major macrovascular complications, such as myocardial infarction (MI), stroke, and peripheral artery disease.
Microvascular complications of diabetes
The microvascular system, which consists of arterioles, venules, and capillaries, is the basic functional unit of the cardiovascular system. These blood vessels have different cellular components and distribution than macrovessels, and have mechanisms regulating permeability and blood flow to achieve proper nutrient delivery and to maintain adequate systemic blood pressure (83, 187, 225). The microvascular complications of diabetes are the leading cause of blindness and end-stage renal disease in developed countries (212). Additionally, around 60% of patients develop neuropathy, which together with PAD can account for around 50% of non-traumatic amputations in the US (212).
Diabetic retinopathy
Diabetic retinopathy is the leading cause of visual impairment in working-age adults worldwide, and is one of the major microvascular complications of diabetes. It affects the retinal vasculature, leading to complications such as microaneurysms, cotton wool spots, abnormal angiogenesis, capillary dropout, and edema (47). Almost 100% of patients with insulin-dependent diabetes mellitus (IDDM) will develop some form of retinopathy that will continue to progress even after tight glycemic control (145), while retinopathy will develop in >60% of individuals with Type 2 diabetes (185). During the time between the initial diagnosis of diabetes and the clinical detection of retinopathy, which can be in years, various irreversible physiological and biochemical changes occur (145). This phenomenon of the continual progression of retinopathy in spite of tight glycemic control indicates a point of no return, possibly due to a ‘metabolic memory’, in which vascular and retinopathy occurrence is virtually inevitable (9, 571). Most therapies currently being developed are directed towards limiting diabetic retinopathy progression; however, the understanding of the molecular mechanisms leading to the pathology is limited, which could hinder the development of better therapies aimed at preventing its occurrence and progression.
Historical perspective of diabetes and associated retinopathy
A 3rd dynasty Egyptian papyrus written in 1552 BC by the Egyptian physician Hesy-Ra described frequent urination as a characteristic of a disease that was later identified as diabetes, with this being considered the oldest record of the disease. In ca.1500 BC, early Hindu writings mentioned an emaciating disease (possibly diabetes) where ants were attracted to the urine of those afflicted. Around 1000 years later, the link between obese individuals and high sugar content in their urine was established. The term diabetes, which originated from the Greek diabaino, was first introduced by the renowned Pneumatic School physician Aretaeus of Cappadocia, who lived in Alexandria and Rome in the 2nd century AD (291) and who was the first to distinguish between diabetes mellitus and diabetes insipidus (332). However, some records indicate that the term “diabetes” might have been coined much earlier by Apollonius of Memphis in 230 BC (223). Diabetes was later described by 1st century Greeks as a melting disease where the body was thought to be converted into urine.
However, it was not until the mid-19th century when the link between diabetes and eye disease was established, starting with the findings by the French ophthalmologist Apollinaire Bouchardat. Visual loss progression was observed and reported in patients with diabetes even with the absence of cataract by Bouchardat in 1846 (82, 285). Vision was found to be improved through exercise and diet (291). In the now famous painting by the Austrian ophthalmologist Eduard Jäger, diabetic macular changes were recorded with the help of the ophthalmoscope he built in 1855 (180). By the second half of the 19th century, several studies further confirmed the link between maculopathy and diabetes. In 1869, an article published by Henry Noyes supported this link (432), which was further described by Edward Nettleship’s paper in 1872 detailing the first proof of diabetes-induced histopathological changes to the macula (291).
In 1876, Wilhelm Manz reported the retinal changes occurring during the proliferative stage of diabetic retinopathy, including degeneration of the optic disk, vitreous hemorrhage, and retinal detachment (183), which further supported the link between diabetes and retinal damage. By the end of the 19th century, an extensive description of the characteristics of diabetic retinopathy was available; however, the mechanisms and explanations for these characteristics were lacking. The first classification of diabetic retinopathy into stages was by the German ophthalmologist Julius Hirschberg, where he classified the pathology into 1) ‘retinitis centralis punctuate’, 2) hemorrhagic form, 3) retinal infarction, and 4) hemorrhagic glaucoma (569). Additionally, the role of retinal microvascular dysfunction in diabetic retinopathy was established by the English ophthalmologist Arthur Ballantyne (41). These and other early discoveries of the link between diabetes and changes to the retina have initiated and contributed substantially to the current understanding of diabetic retinopathy.
Anatomy
Eye
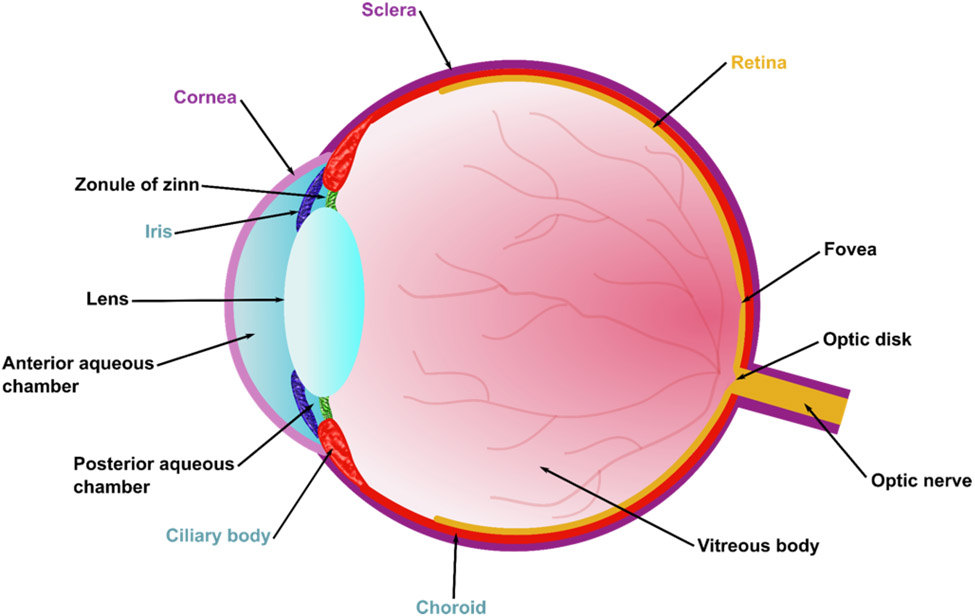
The eye is one of the most complex organs of the body. It contains multiple sections, with tissue that is among the most metabolically active and vascularized in the body. The eye can be divided into three main layers (Figure 2): (1) the sclera and the cornea that form the external layer, (2) the intermediate layer that is divided into an anterior part encompassing the iris and ciliary body, in addition to the posterior part encompassing the choroid, and (3) the retina, which forms the internal layer. Additionally, three fluid chambers exist in the eye: (1) the anterior aqueous chamber (filled with aqueous humor), (2) the posterior aqueous chamber (also filled with aqueous humor), and (3) the vitreous chamber (filled with vitreous humor). Anterior to the vitreous humor sits the lens, a transparent body that is suspended by ligaments known as the zonula of zinn. The lens can change its shape in order to focus light by the contraction of the ciliary muscles that control the zonula of zinn. Once the focused light hits the retina, it is converted to neurochemical signals that are transported to the brain via the optic nerve to be processed.
Figure 2. Anatomy of the eye.
Ocular tissue can be divided into three layers: 1) an outer layer consisting of the sclera and the cornea, 2) an intermediate layer consisting of the iris, the ciliary body, and the choroid, and 3) an internal layer consisting of the retina.
Retina
The retina is a thin tissue that is comprised of a complex network of neurons and supporting cells that provide sensory function, and is considered part of the central nervous system (108). Moreover, the retina shares the high metabolic demand of the brain, and thus, it is one of the body’s most metabolically active tissues (108, 579). Additionally, the blood-brain barrier (BBB) and the BRB share many other characteristics (390).
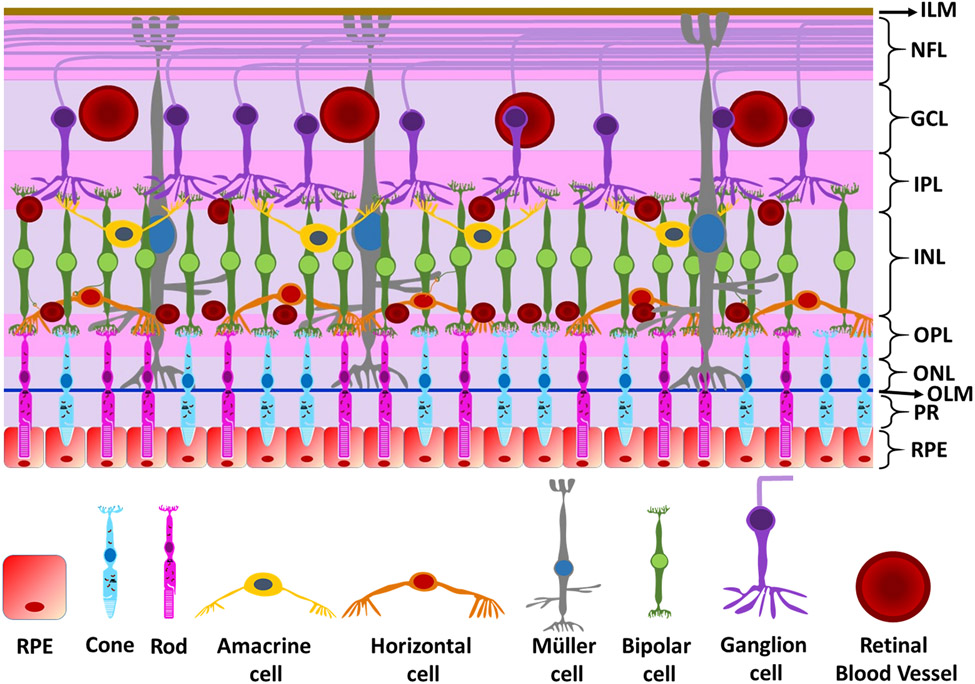
The retina can be divided into distinct layers, three containing nerve cell bodies, and two containing neuronal synapses (Figure 3). Beginning from the outermost layers (farthest from the light source and adjacent to the choroid and the sclera), the following are the layers of the retina:
Figure 3. Layers of the retina.
The layers of the retina starting from the outermost to the innermost layer are the 1) retinal pigmented epithelium (RPE), 2) photoreceptors (PR), 3) outer limiting membrane (OLM), 4) ) outer nuclear layer (ONL), 5) outer plexiform layer (OPL), 6) inner nuclear layer (INL), 7) inner plexiform layer (IPL), 8), ganglion cell layer (GCL), 9) nerve fiber layer (NFL), and 10) inner limiting membrane (ILM).
1. Retinal pigmented epithelium (RPE)
The RPE contains highly specialized neuroectodermally-derived columnar/cuboidal epithelial cells that produce and store melanin, and sits between the neurosensory retina and the choroid (504). It has essential roles in maintaining visual functions including phagocytosis, limiting the scattering of light due to melanin, maintenance of the outer retinal barrier, and transport of metabolites (503, 504).
2. Photoreceptor Layer - outer and inner segments
Photoreceptors are neuroepithelial cells known as the rods and cones which are part of the photoreceptor layer (outer and inner segments) and the outer nuclear layer (ONL) (381). Adjacent to the RPE cells is the rod outer segment which contains the protein rhodopsin, which is responsible for detecting photons. The metabolic demands required for the release of the rod neurotransmitter glutamate are met by the high density of mitochondria found in the inner segment of the rods (504). In the human retina, rods constitute 95% of the photoreceptors, and provide high sensitivity under dark conditions (11). Cones, on the other hand, constitute only 5% of the human retina (11). Cones facilitate high acuity color vision during the daytime, and are not evenly distributed throughout the retina, where they reach a maximum density in the fovea (110), and like rods, release glutamate in the outer plexiform layer (OPL) (381).
3. Outer limiting membrane (OLM)
The OLM is a basal lamina layer that separates the rod and cone bodies from their nuclei. It provides structural support to the retina via its mechanical properties. The junctional proteins occludin, zonula occludens-1, and junctional adhesion molecules (JAMs) have been found to be expressed at the OLM, suggesting it may play a role in the maintenance of the retinal blood barrier (441).
4. Outer nuclear layer (ONL)
The ONL contains the cell bodies, which contain the nuclei of rods and cones (381).
5. Outer plexiform layer (OPL)
The OPL lies between the outer nuclear layer and the inner nuclear layer. Synapses between the dendrites of horizontal cells, bipolar cells, and rod and cone axons are formed within this layer (379). These synaptic interactions lead to the formation of the ON and OFF pathways, where visual signals are split into two channels for detection of objects that are either lighter or darker than the background, thus providing visual contrast (112).
6. Inner nuclear layer (INL)
The INL contains cell bodies of horizontal cells, bipolar cells, and amacrine cells. Horizontal cells are excitatory interneurons that connect bipolar cells, and are responsible for the ability to adjust vision under light and dark conditions (84). Bipolar cells are excitatory neurons that connect the photoreceptors to ganglion cells, and transform the photoreceptor signals in term of polarity, chromatic preference, and kinetics (167). Amacrine cells are inhibitory interneurons that connect bipolar and ganglion cells; they mainly feedback-inhibit bipolar cell terminals, and feedforward-inhibit ganglion cells (40, 84, 112).
7. Inner plexiform layer (IPL)
The IPL is the location where dendrites of ganglion cells, dendrites of amacrine cells, and axons of ganglion cells interact and form synapses with each other (487, 504).
8. Ganglion cell layer (GCL)
The GCL contains the cell bodies of the ganglion cells, which are the output neurons of the retina. The intermediate bipolar and amacrine neurons transmit signals from the photoreceptors to the ganglion cells, where image-forming and non-image-forming signals are transported to the brain via their axons through the optic nerve (487, 507).
9. Nerve fiber layer (NFL)
The NFL contains unmyelinated ganglion cell axon fibers, which run through the vitreal surface of the retina toward the optic disc, penetrating the sclera, and forming the optic nerve (487, 504).
10. Inner limiting membrane (ILM)
The ILM is composed of astrocytes, footplates of Müller cells, and basal lamina, and is considered the boundary between the retina and the vitreous body (487).
Retinal vascular anatomy
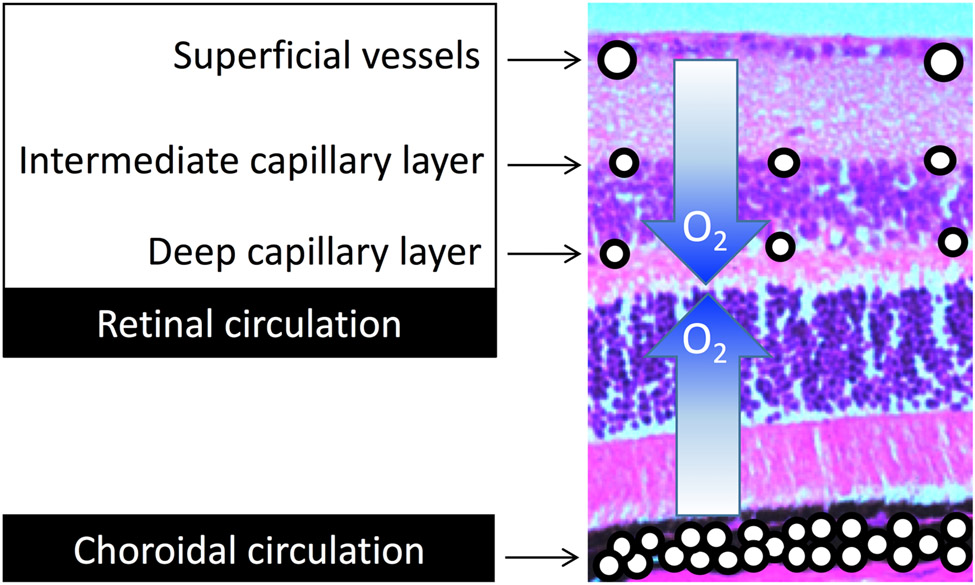
The retinal vascular system is easily accessible making it the most studied vascular system in the human body (181). Due to the retina’s high metabolic activity as a result of the continual conversion of light into neuronal signals, its oxygen demand is high (even more so in the dark than in the light). To meet this high oxygen demand, and to prevent the interference of light transmission by a dense vasculature, two distinct vascular networks are utilized to supply the retina with nutrients and oxygen: 1) the choroidal microcirculation that branches from the posterior ciliary artery, which supplies the outer one-third to one-half of the retina (Figure 4), and 2) the retinal microcirculation that branches from the central retinal artery, and supplies the inner one-half to two-thirds of the retina.
Figure 4. Oxygen sources to the retina.
The inner half of the retina receives oxygen from the retinal circulation, while the outer half receives oxygen from the choroidal circulation.
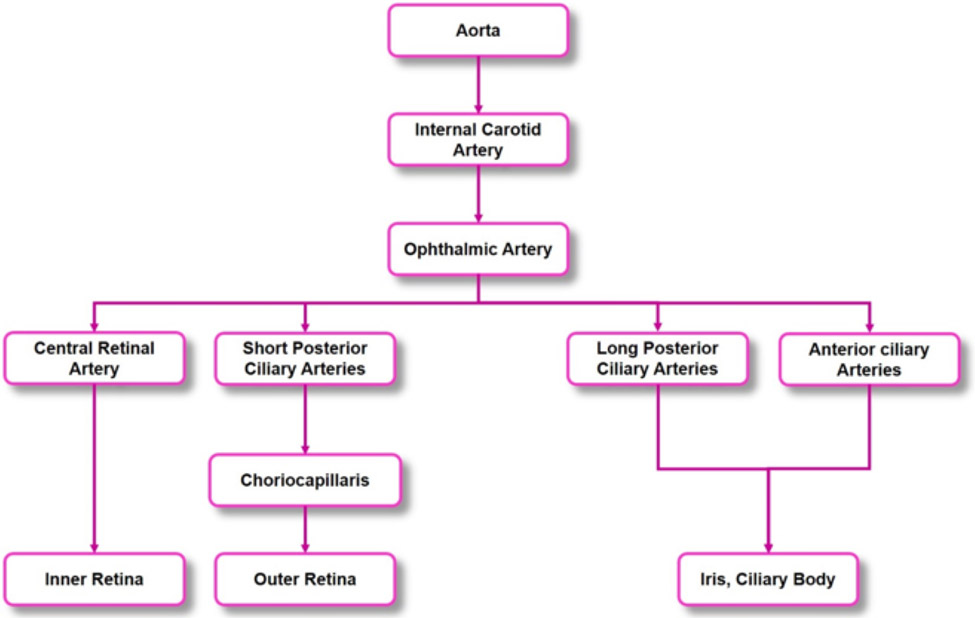
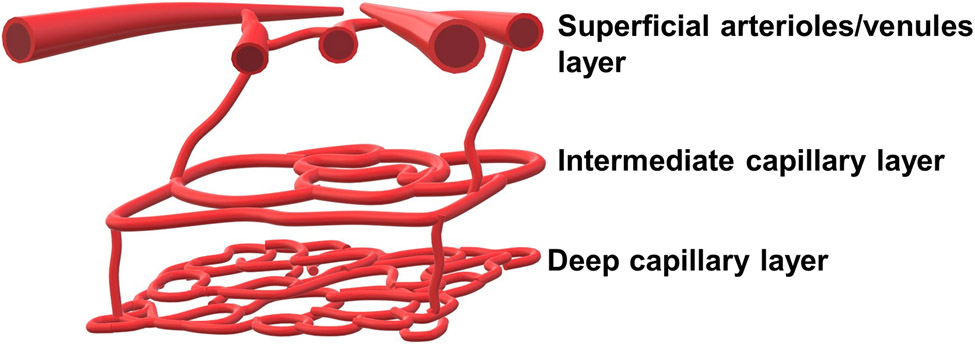
The ophthalmic artery, which originates from the internal carotid artery, branches into the central retinal artery, the short and long ciliary arteries, and the anterior ciliary arteries (Figure 5). The central retinal artery divides into the following four superficial arteriole branches which supply quadrants of the retina: superior nasal artery, inferior nasal artery, superior temporal artery, and inferior temporal artery. The superior and inferior temporal arteries wrap around the thinner avascular macula (236). These superficial vessels branch into the retina creating two distinct capillary layers: the intermediate capillary layer and the deep capillary layer (450) (Figure 6). The superficial vasculature lies in the ganglion cell layer, and contains arterioles, venules, and their branches, mainly supplying the ganglion cells. The arteriolar branches become more narrow as they continue deeper into the retina, where they divide into the intermediate capillary layer. The intermediate capillary layer is a distinct network of capillaries that lies at the border between the IPL and the INL. Capillaries then continue further into the retinal tissue, to finally form the deep capillary layer, which is a dense network of vessels that are highly interconnected, at the border between the INL and the OPL. The architecture results in zones of tissue lacking capillaries, with these zones being between the superficial vessels and intermediate capillary layer, between the intermediate and deep capillary layers, and between the deep capillary layer and the choroid (471). The retinal capillaries drain into the retinal veins, which lie deeper to the retinal arteries, and ultimately drain into the central retinal vein (236).
Figure 5. Arterial branches leading to the eye.
The blood vessels supplying blood to the inner and outer retina, the iris, and the ciliary body originate from the ophthalmic artery, which is a branch of the internal carotid artery. The ophthalmic artery further divides to give rise to the anterior ciliary arteries, the long and short posterior ciliary arteries, and the central retinal artery.
Figure 6. Retinal microvascular layers.
The retinal microcirculatory system can be divided into three distinct layers: 1) the superficial arterioles and venules, 2) the or intermediate capillary layer, and 3) the deep capillary layer.
The inner retina (i.e., NFL to the outer portion of the INL) receives nutrients from the retinal circulation, while the chroidal circulation supplies the outer retina. Arteries exit and venules enter the optic disc in an alternating fashion, with the pattern being radial in rodents (240, 382, 450), but more of a C-shape around the macula in humans. Additional differences found between the human and rodent retina are discussed in greater detail elsewhere (111, 450, 603). Smooth muscle cells surround the retinal arterioles prior to branching into retinal capillaries (540). Retinal capillaries consist of non-fenestrated endothelial cells (109) connected by tight junctions which form the inner blood-retinal barrier (109, 440, 541). These capillary endothelial cells are surrounded by pericytes (540).
Choroid structure and vascular anatomy
With the exception of the endothelium of the blood vessels and innermost layer of Bruch’s membrane, the choroid is derived from neural crest cells. The choroid is a highly vascularized structure that sits on the outer side of the retina with the inner choroid (i.e. Bruch’s membrane) connected to the retinal pigment epithelium (from the ora serrata to the optic nerve), while the outer choroid is attached to the sclera. The choroid has one of the highest flow rates per gram of tissue in the human body, and combined with the anatomical arrangement, the choroid is positioned to meet the metabolic demands of the outer retina and retinal pigment epithelium (226).
Branching off the ophthalmic artery, the short posterior ciliary arteries penetrate the sclera, providing localized blood supplies by forming into fan-shaped lobules. These arteries pierce the sclera to form three layers of the choroidal microcirculatory system (302). The outermost layer is known as Haller’s Layer which contain larger blood vessels surrounded by smooth muscle cells. The middle layer is Sattler’s layer, which contains medium-sized blood vessels. The vessels in Haller’s and Sattler’s layers are not fenestrated (226). The innermost layer is the choriocapillaris and is located adjacent and inner to Sattler’s layer. The endothelial cells of the choriocapillaris are fenestrated with most fenestrations on the inner side, which allows for oxygen and nutrient supply to the outer retina (109, 226). The long posterior ciliary arteries and the anterior ciliary arteries supply the iris and the ciliary body (302). The choroidal circulation is regulated by the sympathetic and parasympathetic nervous systems (133, 369).
Retinal Oxygenation and Metabolism
The main function of the retina is to convert light signals to neuronal signals that can be carried by the optic nerve to the brain for processing. This continual activity necessitates a tight control of retinal blood flow to ensure proper function (mechanisms discussed in a later section), in part through adequate oxygen delivery.
Oxygen tension profile in the retina
The oxygen tension profile from the front to the back of the retina is unique, and is influenced by the presence of the retinal and choroidal microcirculation networks. These vascular networks are critical for sufficient oxygen supply to the retina, but differ in their capillary density, arterio-venous oxygen distribution, and autoregulatory control. The oxygen tension profiles differ among animal species due to variations in retinal and choroidal circulations and retinal thicknesses. For example, the rat and mouse retina have similar capillary layers (i.e. intermediate and deep layers) (645, 646) as found in the human retina, except in the macula, which is the thinnest part of the human retina and does not contain any vasculature. Additionally, the guinea pig does not contain a retinal circulation and receives its entire oxygen supply from the choroidal circulation (647).
The oxygen tension profile of the choroid is highly dependent on the supply coming from the choroid. The choroidal circulation receives 80-85% of ocular blood flow (25), and has one of the highest blood flows per unit mass in the body (25, 219). The choroid supplies 79% of the oxygen consumed by the retina (26), but with a low oxygen extraction (24, 99, 159, 578). Yu et al. (645, 648) measured the retinal oxygen partial pressure in rats and mice using microelectrodes, and observed a significant decrease in the oxygen tension starting from the retinal tissue near the choroid (~45 mmHg), with a decrease in oxygen tension to ~10-15 mmHg in rats (646) and ~4-6 mmHg in mice (645) just anterior to the photoreceptors, consistent with the high metabolic rate of these neurons. Similar trends were observed in pigs and cats (607). This decrease in oxygen tension reflects the high metabolic rate of these neurons, and reflects the inability of the choroidal microcirculation to supply oxygen to the entire inner retina, with the exception of rabbits and guinea pigs. This is evidenced by the oxygen profiles in the inner retina being similar with or without inspiration of 100% oxygen following laser occlusion of the retinal arteries (648).
In contrast to the choroidal circulation, blood flow in the retina is not as high (25); however, inner retinal oxygenation by the retinal microcirculation is vital with the arteriovenous difference in oxygen partial pressure being approximately 40% (252, 578), and with possibly one-third of the oxygen consumed by the retina being supplied by the retinal circulation. To demonstrate the retinal circulation contribution to inner retinal oxygenation, Yu et al. (648) conducted experiments where retinal ischemia was initiated by laser occlusion, followed by oxygen tension measurements, with the oxygen tension decreased to ~0 mmHg throughout the inner retina. By performing this experimental protocol, the researchers were able to model the inner retinal oxygen consumption, which was feasible due to the elimination of flowing vessels that could interfere with this protocol and analysis. The researchers concluded that the inner retinal oxygen consumption is substantial, and equals that of the outer retina. Moreover, the unique distribution of the retinal microvessels and their sparsity compared to the choroidal microcirculation permits light transmission through the retinal layers. However, the sparsity of the retinal circulation can also contribute to its susceptibility to vascular disease (644).
Retinal metabolism
Three dominant layers have been identified as sites of oxygen consumption in the retina, with this identification established with oxygen profile analyses in rats. The three layers include: 1) the photoreceptor inner segment, which contains the mitochondria, 2) the outer plexiform layer, and 3) the deeper region of the inner plexiform layer (644). Moreover, activity of cytochrome oxidase, the enzyme involved in the final step of the mitochondrial respiratory chain, is the most intense in retinal cross-section staining in these three layers (331), which indicates a significant oxygen consumption. This oxygen consumption is needed to produce ATP and facilitate synaptic transmission (648), phototransduction (28), Na-K ATPase activity, and other activities that require significant amounts of energy, including guanosine triphosphate synthesis and the generation of the dark current (607). Glycolysis produces a small amount of energy even though a large amount of glucose is consumed. The transition from steady light to dark increases oxygen consumption with the demand thought to be met by oxidative metabolism instead of glycolysis (28). The energy demands during light flashes, alternating with darkness, are thought to be met by glycolysis (28), which is consistent with an abundance of glycolytic enzymes found in the inner retina (368).
Darkness may induce complete retinal tissue anoxia in some parts of the cat retina, as a result of the increase in oxygen consumption (358). Interestingly, this phenomenon is not a general characteristic of all mammalian retinal tissue, as reported by Yu & Cringle (643), who demonstrated that oxygen tension in the outer retina of dark adapted rats does not decrease below 5 mmHg despite a 50% increase in oxygen consumption. This difference was explained by the increase in oxygen supply by increases in retinal blood flow in dark adapted rats, with this increase also occurring in humans (171).
Retinal and Choroidal Blood Flow
Regulation of retinal blood flow
Regulation of retinal blood flow occurs through local tissue factors, metabolic factors, and physical factors in response to changes in metabolic demands, light/dark transition, and pressure changes (i.e. systemic, intraocular, and perfusion pressure). Although the autonomic nervous system affects blood vessels in extraocular tissue (133), there is no change in retinal blood flow or preretinal oxygen tension following stimulation of the superior cervical sympathetic chain (53). In addition, there is no intraocular innervation of retinal blood vessels by adrenergic, cholinergic, or peptidergic nerves (257, 261, 262, 337, 639) despite the presence of cholinergic receptors (175). However, blood flow to the retina is regulated in part by glial cells that surround the retinal vessels (350), and by the metabolic needs of the retinal neurons being nourished by the retinal circulation. Below we review a number of factors that regulate retinal blood flow.
Regulation of retinal blood flow – blood gas
Oxygen
Oxygen concentrations help regulate retinal blood flow. Zhu et al. (657) demonstrated this regulation in a series of experiments wherein newborn pigs were provided 100% oxygen to breathe during measurements of retinal blood flow. The protocol resulted in hyperoxia and a decrease in retinal blood flow by around 40% within 5-10 minutes. Treatment with inhibitors targeted against endogenous vasoconstrictors such as 20-hydroxyeicosatetraeonic acid (20-HETE), endothelin, and thromboxane attenuated this response; however, antioxidant treatment with catalase and superoxide dismutase had no effect. The vasoconstrictive hyperoxia response also occurs in other animal models (161, 496, 552) as well as in humans (280, 493), where retinal arteriolar diameters and retinal blood flow decrease by as much as 60% as a result of 100% O2 inhalation (280, 493). The response to hyperoxia is important to maintain a constant retinal pO2 (472, 496). In comparison, diabetes-induced decreases in retinal blood flow rate in rodents (decreases of ~33%) have been found to be attenuated not only by targeting the vasoactive mediators thromboxane (343, 344, 622, 626), endothelin (605), 20-HETE (606), and angiotensin II (343), but also by scavenging superoxide with tempol (631), with the latter implicating ROS in retinal blood flow control during diabetes. However, in the diabetic retina, the exact mechanisms by which oxygen levels regulate the vasoconstrictor pathways is yet to be determined.
Not only does hyperoxia decrease retinal blood flow, but the reverse also is true, i.e., hypoxemia (low oxygen) increases retinal blood flow in cats (359), humans (470), and monkeys (161), presumably in an effort to maintain retinal pO2. This mechanism has been shown experimentally when outer retinal oxygen tension decreased while inner retinal oxygen tension was maintained in experimentally induced hypoxia (359, 397).
Carbon Dioxide
Both hypercapnia (high arterial partial pressure of carbon dioxide; PaCO2) as well as hypocapnia (low PaCO2) affect retinal blood flow. In monkeys, retinal blow flow and preretinal vitreous oxygen tension (a measure of inner retinal oxygen availability) have been shown to decrease in hypocapnia (581, 582). Hypercapnia leads to an increase in retinal blood flow with each 1 mmHg rise in PaCO2 resulting in a 3% rise in blood flow (582) and preretinal vitreous oxygen tension in monkeys (581). The hypercapnic response (i.e., increased retinal arteriolar diameter and flow) also has been reported in healthy adult humans (589).
Autoregulation and metabolic regulation
Retinal arterioles can maintain relatively constant tissue perfusion with moderate changes in perfusion pressure, with this mechanism known as autoregulation (133). Autoregulation occurs through a wide range of pressures in the healthy eye (25, 179, 491, 497, 500). Retinal blood flow also is regulated by the metabolic needs of the retina through the release of local factors from vascular endothelial cells and neural tissue surrounding the vessels (387, 473).
Retinal vascular control of flow through autoregulation and metabolic regulation is achieved by a complex system of vasodilators and vasoconstrictors, including nitric oxide (NO), prostacyclin, thromboxane A2 (TxA2), endothelin-1, and angiotensin II (Ang II) (229, 473). These potent vasoactive factors are produced by endothelial (and other) cells, and can modulate vascular tone by affecting vascular smooth muscle cells and/or pericytes. Thus, the retinal endothelium plays an important role in regulating retinal blood flow, which under pathological conditions can be disrupted due to endothelial dysfunction.
Nitric Oxide
Nitric oxide (NO), also known as endothelium-derived relaxing factor (EDRF) (520), is synthesized by NO synthase (NOS), and is formed as a result of the oxidation of L-arginine by oxygen, resulting in NO and L-citrulline (186). NO synthase is expressed in several retinal cells (81, 633), and has three isoforms: inducible NOS (iNOS), neuronal NOS (nNOS), and endothelial NOS (eNOS) (186). Non-vascular retinal cells express nNOS (123, 217, 316, 443, 590, 633), while eNOS is expressed in retinal and choroidal endothelial cells and retinal pericytes (81, 315, 388). In the non-diseased retina, iNOS is not expressed (6); however, during hypoxia (295) and in the retina of diabetic patients (6), iNOS is expressed in various retinal cell types.
Regulation of vascular tone is one of the main roles of NO. Released NO regulates vascular relaxation by modulating intracellular level of ions such as Ca2+ and K+ by activation of ion channels through activating guanylyl cyclase/cyclic guanine monophosphate (GC/cGMP) in smooth muscle (242). Many vasodilators including adenosine (114, 241, 249, 459, 592), acetylcholine (86, 298, 630), bradykinin (39, 106, 115, 122, 246, 410), histamine (309, 334, 351, 445), lactate (61, 199, 202, 247), and norepinephrine (283, 306) exert their effects by the release of NO.
Ocular blood flow and vasodilation depend on NO derived from the NO synthases in the retina. A variety of early studies investigating ocular blood flow found decreases following administration of Nitro-L-arginine (L-NA) (147), NG-nitro-L-arginine methyl ester (L-NAME) (140, 219, 246, 247, 249, 304, 323, 410, 411, 543), and NG-monomethyl-L-arginine (L-NMMA) (34, 220), suggesting a significant role for NOS in ocular blood flow. More specifically to the retina, acetylcholine-induced relaxation in retinal arterioles is attenuated with L-NMMA treatment (48, 637), and the effects of nicotine, histamine, and substance P on retinal vascular tone is mediated by the release of NO (48, 311, 576).
NO produced from eNOS plays an essential role in maintaining vascular functions. Laspas et al. have examined the different NOS isoforms in the ex vivo reactivity of the ophthalmic artery to acetylcholine (335), with their results pointing to the importance of eNOS, but also to compensatory NOS-independent vasodilatory pathways with a chronic loss of eNOS. Further downstream in the retinal arterioles, similar results have been found with regards to the importance of eNOS in endothelium-dependent vasodilation (208, 209), and also with regards to the upregulation of compensatory pathways in the absence of the enzyme (209).
Light flicker is known to dilate retinal arterioles, and although the precise mechanism is not completely understood, the resulting functional hyperemia (68, 147-149, 200, 255, 320, 464) is thought to be mediated by NO. Retinal NO levels increase following flicker stimuli (68, 147), as is the blood flow response in the optic nerve head (68, 320). Ocular vascular responses to light flickering is suppressed by NOS inhibition with L-NAME (68, 320, 642), L-NMMA (134), NG-nitro-L-arginine (L-NNA) (68), and N(ω)-propyl-L-arginine (L-NPA) (642).
Studies have indicated that retinal neural activity and NO contributes to the light flicker response (320, 494, 546), which is modulated by glial toxin (148, 543, 546) or nNOS inhibitors (543, 642). Moreover, the amount of NO in retinas from eNOS-deficient mice has been found to be similar to that in wild-type C57BL/6 mice (possibly due to compensatory production of NO mentioned earlier), and distribution of nNOS in the deep retinal capillary plexus is increased in eNOS knockout mice (18).
Prostaglandins
Prostaglandins, which can be synthesized by retinal endothelial cells (499), are produced as a result of the metabolism of arachidonic acid by cyclooxygenase, leading to the formation of four main prostaglandins: prostaglandin E1 (PGE1), prostaglandin F2α (PGF2α), prostaglandin D2 (PGD2) and prostacyclin (PGI2) (489). Prostaglandins have been shown to regulate retinal vascular tone through complex mechanisms, where they can act as vasodilators or vasoconstrictors. In studies on anesthetized rabbits, systemically administered PGE1 and PGF2α caused retinal vasodilation (549). However, these prostaglandins caused vasoconstriction of isolated bovine retinal arteries and miniature pig retinal arterioles (422, 470). Moreover, PGE1 and PGI2 increased retinal blood flow in rats when injected intravenously (398).
Thromboxane
Thromboxane A2 (TxA2), also synthesized as a result of arachidonic acid metabolism by cyclooxygenase (322), binds to the thromboxane-prostanoid (TP) receptor (584). Sources of TxA2 include platelets (433) and leukocytes (254). TxA2 does not play a role in retinal blood flow in non-diabetic mice or rats but its inhibition restores retinal blood flow in hyperglycemic rodents (343, 344, 622).
Endothelin
Endothelin-1 (ET-1), also known as endothelium-derived constricting factor (EDCF) (588), is the most potent vasoconstrictor that has been discovered to date (636). ET-1 is mainly synthesized by endothelial cells, however, other cell types have been shown to synthesize the mediator, including vascular smooth muscle cells, macrophages, neurons, and leukocytes (297). ET-1 is part of the endothelin family that also include ET-2 and ET-3, and is synthesized by the cleavage of preproendothelins to big endothelins, which is further cleaved to form ET-1 (275). Endothelin-1 binds to endothelin-A (ETA) and endothelin-B (ETB) receptors, both of which are expressed on retinal arterioles (245). ET-1 binding to receptors on vascular smooth muscle induces vasoconstriction. Although activation of ETB receptors on the endothelium has the capability of inducing vasodilation mediated by NO (518), ETB receptors have been found to play a relatively insignificant role in the porcine retinal circulation (88, 466). Under basal conditions, ET-1 expression is reduced by NO and PGI2 (228, 482); however, shear stress, norepinephrine, cytokines, and angiotensin II increase its expression (378). In healthy adult humans, retinal vessel diameter, blood flow, and velocity are significantly reduced with the administration of ET-1 (463). Moreover, dose-dependent vasoconstriction occurs in rat (70), rabbit (566), and cat (221) retinal arterioles with intravitreal injection of ET-1.
Angiotensin II
Angiotensin II is a hormone produced when the angiotensin converting enzyme (ACE) converts angiotensin I to angiotensin II. Angiotensin II binds to AT1 receptors resulting in vasoconstriction, and to AT2 receptors resulting in vasodilation. Ocular tissue contains the components required to produce its own angiotensin II (116, 117). In addition, the retinal vasculature contains ACE, AT1 receptors and AT2 receptors (174, 176, 408, 509, 611).
Lactate
Aerobic and anaerobic glycolysis produces lactate in the retina with anaerobic being greater than aerobic production. Multiple cell types in the inner and outer retina produce lactate in the normoglycemic state (615). When energy supply is sufficient, lactate produces constriction in the retinal microvasculature; yet lactate produces dilation of the retinal microvasculature during hypoxic conditions (634). Lactate-induced dilation of isolated porcine arterioles has been found to be mediated by NO synthase, activation of guanylyl cyclase, and KATP channels (247).
Adenosine
Adenosine is formed from the degradation of adenosine triphosphate (ATP). Adenosine is a vasodilator that has been shown to function in the retinal circulation as part of the autoregulatory process (60, 213, 214). Retinal vessel responses occur from adenosine release from neural tissue on the outside of the vessel during hypoxia or ischemia (21). The response to adenosine occurs through the A2 receptor which stimulates the opening of KATP channels and release of NO (249, 281, 352).
Retinal arterio-venular communication
Arteriolar diameter can be controlled by venules that are closely paired to arterioles flowing in countercurrent directions (237, 251). In this mechanism, it is thought that vasoactive metabolites from the venules diffuse to the closely-paired arterioles and cause vasodilation or vasoconstriction (251). In the human retina, arterioles and venules are found in this type of parallel, countercurrent orientation, but whether or not this arrangement helps control arteriolar perfusion has yet to be determined. In the rodent retina, arterioles and venules extend out from the optic disk in a more radial than parallel fashion, although presumably, diffusive exchange could still occur between venules and arterioles having smallest angles between the two, with data from a few studies supporting this possibility (343, 344, 622).
Retinal blood flow - light conditions
Retinal blood flow responses to light/dark adaptation have been varied. During dark adaptation, flow velocity increases in retinal arteries and veins in healthy subjects when measured with laser Doppler velocimetry (171, 492), and retinal blood flow also increases in the dark when measured using a microsphere perfusion assay (53). However, others have reported no change during dark adaptation in flow velocity, using a laser Doppler flowmeter (495) – or in arterial diameter changes, using a scanning laser ophthalmoscope (45).
Regulation of choroidal blood flow
Choroidal blood flow is one of the highest per gram of tissue in the body (25, 219) and supplies a large fraction of total ocular perfusion (25). Despite the high blood flow, oxygen extraction is only 3-4% (24, 159, 578). The autonomic nervous system controls choroidal blood flow (52, 133): sympathetic nervous system innervation occurs through the superior cervical ganglion, and uveal blood vessels constrict upon stimulation, resulting in a decrease in choroidal blood flow (23, 52, 133, 369). The choroidal response to sympathetic stimulation is thought to be protective via the autoregulation of blood flow and perfusion pressure (53).
The parasympathetic nervous system innervation occurs through the facial nerve, oculomotor nerve, and the trigeminal nerve (ophthalmic and maxillary divisions) (133, 369), with choroidal blood flow increases occurring upon stimulation of those nerves (555-557). Environmental light increases blood flow in the choroid (192), with the opposite occurring in the dark (193). Maintenance of outer retinal temperature appears to be an important role of the choroidal blood flow response to parasympathetic nervous system stimulation (454, 455).
Choroidal blood flow exhibits autoregulation at intraocular pressures (IOP) below 25 mmHg when the perfusion pressure (mean arterial pressure - intraocular pressure) exceeds 40 mmHg (305). When IOP is held constant at resting or elevated levels, choroidal blood flow becomes more linear when mean arterial pressure is decreased (i.e., less autoregulation) (305). As IOP continues to rise to levels 3-fold above control, choroidal blood flow decreases (25). Autoregulation of the choroid occurs through the myogenic mechanism (303, 305), which is based on the law of Laplace: vascular wall tension (T) = transmural pressure (P) × vessel radius (r). As transmural pressure increases, vessel diameter will decrease in an effort to maintain wall tension, resulting in the maintenance of blood flow.
The blood retinal barrier (BRB)
The retina is part of the central nervous system, and its vascular system shares the same characteristics as the brain vasculature. The blood-retinal barrier (BRB), which can be compared in its structure and function to the blood-brain barrier (BBB), is a physiological barrier that limits and controls the circulating blood components from reaching the surrounding tissue. A compromised BRB can lead to various retinal degenerative diseases and blindness.
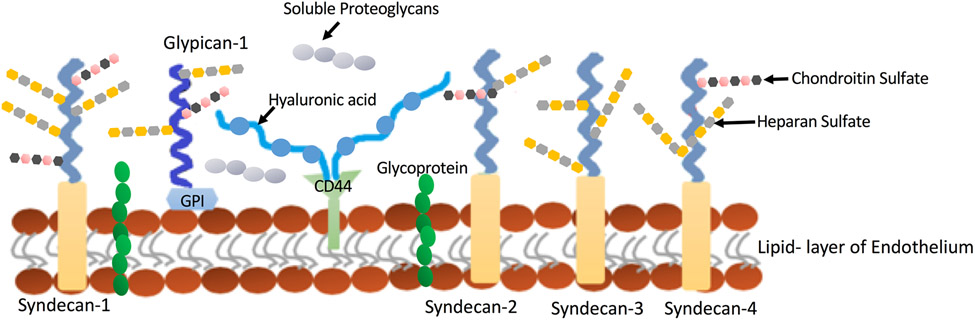
The blood retinal barrier (BRB) can be divided into two components, the inner (iBRB) and outer (oBRB). Adherens and tight junctions between adjacent endothelial cells, and possibly the glycocalyx, form the iBRB. On the other hand, tight junctions between the retinal pigmented epithelial cells (RPE), which segregate the choroid from the neuronal retina, form the oBRB. Occludins, zonula occludens (ZO-1, -2, and -3), and claudins are specialized proteins that are components of tight junctions, and regulate the transport of molecules and solutes through the RPE and endothelial cell layers (55, 301, 390).
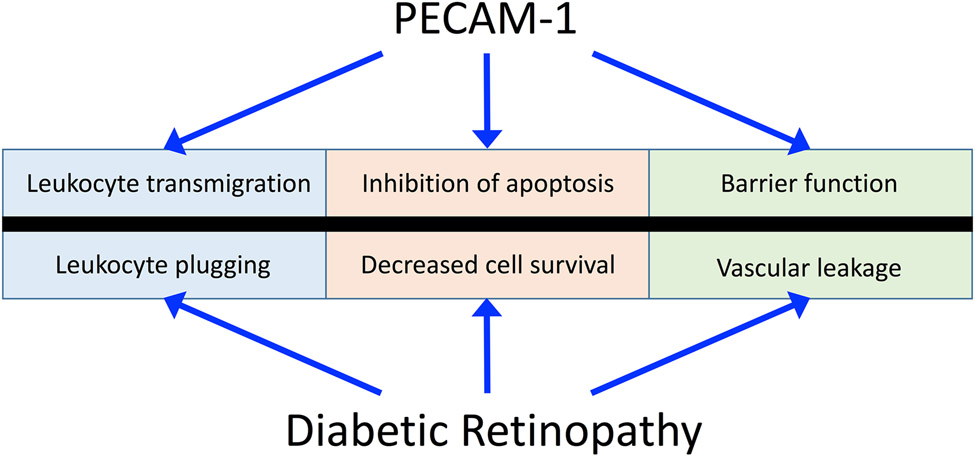
Although tight junctions are common between retinal endothelial and epithelial cells, their organization, distribution, and composition are different between the two cell types. In epithelial cells, tight junctions are localized to the apical side of the cell, while tight junctions in endothelial cells are distributed between gap and adherens junctions. In endothelial cells, adherens junctions are composed of a complex of proteins that anchor to the cytoskeleton and link cells to each other. These proteins include vascular endothelial cadherin (VE-cadherin), catenins, and plakoglobin (132). Moreover, cell-cell junctions contain additional adhesion molecules, such as platelet endothelial cell adhesion molecule-1 (PECAM-1), which helps in the maintenance of retinal endothelial barrier integrity (129). In addition to cell adhesion, these proteins also contribute to cell function by acting as scaffolds, and by participating in cell signaling pathways, contact inhibition, cell growth, and apoptosis (130, 269, 270, 418, 583).
Mechanisms Contributing to Diabetic Retinopathy
Overview of diabetic retinopathy as a microvascular pathology
Although recent work has indicated that retinal neuronal changes may occur even prior to overt microvascular pathology in diabetic retinopathy, the major changes in the vasculature have justified the consideration of diabetic retinopathy as a microvascular disease. Several events occurring in diabetic retinopathy include a breakdown of the blood-retinal barrier, vascular cell death, and leukocyte plugging of capillaries with increased vascular permeability being one of the first observations in diabetic retinopathy during the non-proliferative stage. Leaky vessels lead to the accumulation of fluid in retinal tissue and the development of macular edema, the clinical feature most clinically associated with blindness (145). In addition, many studies have indicated an increase in leukocyte adhesion in the diabetic retina of animal models (mice, rats) and in humans. The plugging of vessels by leukocytes limits the oxygenation of the tissue and vessel wall, causing the formation of acellular capillaries that are no longer perfused. The decrease in blood flow and increased inflammation will stabilize hypoxia-inducible factor-1α (HIF-1α) leading to its translocation to the nucleus and its dimerization with HIF-1β, and binding to the hypoxia response element on the promoter sites of important genes such as the one encoding for vascular endothelial growth factor (VEGF) (522). VEGF will promote uncontrolled angiogenesis in the inner retina with abnormal proliferation. In addition, these new blood vessels can be leaky and fragile leading to edema and microhemorrhages in the more serious proliferative stage of diabetic retinopathy.
Stages of diabetic retinopathy
There are two classifications of diabetic retinopathy: non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). Non-proliferative diabetic retinopathy can be further subdivided into three phases depending on the severity of the complications: mild, moderate, and severe NPDR. These stages represent progressive damage to the retinal microvasculature that contribute to the pathology of diabetic retinopathy.
Non-proliferative diabetic retinopathy
NPDR is marked by various retinal microvascular complications that can be observed clinically, such as intraretinal hemorrhages, microaneurysms, cotton wool spots, and retinal tissue edema due to increased retinal vascular permeability. Additionally, acellular retinal capillaries start to develop due to pericyte and endothelial cell loss. Acellular capillaries are non-perfused, and initially occur in few capillaries, thus have no clinical consequences. However, as the disease progresses and more acellular capillaries develop, hypoxia will become more severe leading to localized ischemia triggering the release of growth factors and the initiation of abnormal neovascularization.
Proliferative diabetic retinopathy
PDR is the advanced and more severe stage of diabetic retinopathy and characterized by the development of new blood vessel growth in the optic disc or elsewhere in the retina. However, these blood vessels are leaky and fragile, causing worsening of intraretinal hemorrhages and microaneurysms (632) and damage to the retina. The new blood vessels can penetrate the vitreous and become leaky, leading to vitreous hemorrhages and retinal detachments (632). It is thought that hypoxia leads to the release of growth factors that promote neovascularization (618); however, there is evidence that hypoxia does not occur early in the diabetic retina (338, 624). Additionally, neovascularization limits the amount of light reaching the cone and rod layers, which further impairs vision.
Initially, the new vessels are asymptomatic, with little to no hemorrhage, and usually originate around the area of the optic disk (119, 570). These new blood vessels form networks and loops, and cross arterioles and venules in the underlying retina, making them distinguishable from other retinal blood vessels (121). Mild thickening of the optic disk and the surrounding retinal tissue also occurs due to vascular leakage and edema (121). As the condition progresses, increased hemorrhage and tissue damage occurs. Moreover, white delicate fibrous tissue starts to form adjacent to the new vessels, forming what are known as fibrous vessels (502). The new blood vessels enter a cycle of proliferation and regression, leaving behind the fibrous tissue that creates traction across the retina (121).
An additional complication of diabetic retinopathy is the weakening of the attachment between the vitreous and the inner limiting membrane of the retina (92, 416). Under normal conditions, collagen, laminin, and fibronectin anchors the posterior vitreous to the inner limiting membrane, however, under hyperglycemic conditions, adhesion between the retina and posterior vitreous weakens, leading to posterior vitreous detachment (PVD) (17). Fibrous vessels and tissue are attached to the vitreous, leading to retinal tearing, hemorrhage, and damage due to PVD.
Current therapies of diabetic retinopathy
Tight glucose control in individuals with diabetes provides some benefits such as delayed onset of retinopathy, slowed progression of retinopathy (414), and improved retinal vascular permeability (56). However, despite the benefits, the risk of developing retinopathy is not eliminated (414) with intensive insulin therapy. Interestingly, in diabetic individuals with mild retinopathy, intensive insulin therapy leads to transient worsening (414) that is mediated in part by HIF-1α upregulation of VEGF (468). Even so, with proper medical supervision, the benefits of intensive insulin control outweigh the risk of hypoglycemia (120, 414).
Panretinal photocoagulation, vitrectomy, and VEGF inhibitors are therapies that are used in the treatment of PDR (189). Panretinal photocoagulation is thought to decrease upregulation of growth factors such as VEGF by ablating regions of ischemia in the peripheral retina (94, 632). A complete review of current therapies is beyond the scope of this review with more comprehensive reviews found elsewhere (58, 94, 178).
While intensive insulin therapy has some benefits and multiple therapies exist for individuals once proliferative diabetic retinopathy occurs, there is no cure for diabetic retinopathy or therapy that prevents its development.
Retinal vascular complications in diabetic retinopathy
Hyperglycemia initiates a destructive cascade of events leading to retinal microvascular alterations. These alterations affect the integrity and functions of the retina and lead to eventual vision loss. By the time microvascular alterations are clinically observable, significant and sometimes irreversible damage has already occurred. Thus, the detection of early molecular changes is important to control the progression of diabetic retinopathy.
Microaneurysms
During the mild phase of NPDR, saccular structures outpouching from capillary, known as microaneurysms, start to form. Microaneurysms are the earliest manifestation that can be observed clinically, and can be a result of structural weakening and modification of the capillary wall. Although the exact mechanisms leading to the development of microaneurysms are not fully understood, studies have speculated that they may arise as a result of pericyte loss and subsequent capillary wall weakening (188). Microaneurysms start initially as hypercellular structures, with the lumen filled with erythrocytes and thrombi, and progress to acellular microaneurysms (650). Microaneurysms can leak plasma, contributing to retinal edema and thickening.
Pericyte dropout
One of the earliest pathological changes in diabetic retinopathy is pericyte loss in the retina (98). As retinal microvessels start to become more tortuous, early signs of pericyte dropout can be observed as empty pockets in the capillary basement membrane due to their loss (98, 232). In addition to pericytes, endothelial cells also are lost, leading to the development of acellular capillaries (188). The mechanism for endothelial and pericyte loss is still under investigation; however, many studies implicate the activation of apoptotic and necrotic pathways due to hyperglycemia, and the accumulation of toxic byproducts leading to cell death (354, 393, 580).
Pericytes are supporting mesenchymal contractile cells that surround capillaries, and are derived from progenitor mesenchymoangioblast cells analogous to smooth muscle cells in larger vessels, and thus, may play a similar role (47). Due to their role in regulating capillary function, pericytes share a basement membrane and stay in close contact with endothelial cells in the capillaries. Additionally, pericytes exist in a 1:1 ratio with endothelial cells in the retina, which is considerably higher than the ratio in the brain, perhaps indicating the involvement of the cells in the strong blood-retinal barrier (231). Multiple factors facilitate the communication between pericytes and endothelial cells, including molecules such as platelet-derived growth factor-β1 (PDGF-β1), sphingosine-1-phosphate (S1P), angiopoietin, and transforming growth factor-β1 (TGF-β1) (169, 231, 233, 264, 318). Pericyte apoptosis and dropout have been observed under pathologic conditions such as hypertension, hyperglycemia, and accumulation of advanced glycation end products, as well as with basement membrane thickening (47, 231, 587). Additionally, due to their role in regulating endothelial cell proliferation and the BRB, pericyte loss could lead to the pathologic angiogenesis of retinal capillaries, as well as the breakdown of the blood-retinal barrier.
Cotton wool spots
Cotton wool spots indicate ischemic tissue in the nerve fiber layer due to occlusion of retinal microvessels (384) and consist of axoplasmic accumulations from nerve fiber axons. Axoplasmic transport, also known as axonal flow, occurs bidirectionally between the soma (neuronal body) and the synapses transporting organelles and cellular components (385). In undamaged segments of the axon, axonal transport continues, leading to terminal swelling of the damaged axon, and the formation of cytoid bodies, which are the histological hallmark of cotton wool spots (33, 385).
Blood-retinal barrier (BRB) breakdown
One of the early events occurring in NPDR is BRB breakdown and hyperpermeability, which sometimes can precede any visible clinical manifestations of retinopathy (188, 650). BRB breakdown leads to capillary leakage and the accumulation of plasma components in retinal tissue causing increased thickening and visual impairment. Hyperglycemia-induced retinal endothelial dysfunction is a significant contributor to the disruption of BRB, due to the vital role that the retinal endothelium plays in the maintenance of BRB integrity. The molecular mechanisms leading to BRB breakdown are multifactorial and complex, with many contributing factors such as increased VEGF levels (406, 612, 619), leukocyte adhesion (286), growth factors (30, 235), cytokines (287), inflammation (523), and a reduction in tight junction proteins (30, 235). These factors, as well as the influence of the endothelial glycocalyx, will be discussed more in detail later in this review.
Molecular mechanisms leading to the pathogenesis of diabetic retinopathy
The development and progression of diabetic retinopathy can be associated with multiple pathological factors, such as hyperglycemia and genetic predisposition (13, 73). Some of the major mechanisms that have been reported in diabetes-induced retinal stress leading to microvascular damage and retinopathy include: 1) oxidative stress, 2) the polyol pathway, 3) protein kinase C (PKC) activation, 4) non-enzymatic glycation, 5) inflammation, and 6) genetic factors. These mechanisms lead to an increased expression of VEGF (7, 59, 124, 375) prior to development of proliferative retinopathy (29), stimulating the growth of new vessels, increased vascular permeability (278), and leukocyte activation and adhesion (278, 288). The next sections of this review will cover the hyperglycemia-induced enzymatic and non-enzymatic changes, and the associated pathophysiological alterations in the diabetic retina.
Glucose transport in diabetic retinopathy
Hyperglycemia is a causative factor in developing microvascular complications in diabetes (1, 195, 414). Glucose, used by retinal neurons in metabolism, has to pass through the endothelial cells that form a barrier between plasma and the underlying tissue. The correlation between poor glycemic control and diabetic retinopathy severity points to the role of chronic high glucose exposure in the retinal endothelium (313). The endothelial cells themselves are sensitive to increases in plasma glucose, with a lack of insulin regulation of glucose transport (32), and with glucose uptake by the endothelium proportional to plasma glucose concentrations (66). Depending on the capillary bed, glucose can either pass through the endothelial cell junctions (with this transport being increased in the diabetic retinal vasculature) or by the specialized glucose transporter GLUT1. The GLUT1 transporter is an insulin-independent, constitutive transmembrane protein that belongs to the major facilitator superfamily (MFS) that is heavily expressed on endothelial cells, and is the primary glucose transporter in the brain and the retina (156, 534, 640).
The multifactorial nature of glucose transport regulation and GLUT1 expression under hyperglycemic conditions need to be considered when discussing the pathological changes occurring in the retinal microvasculature. Multiple factors can regulate the expression of GLUT1, such as growth factors, hypoxia, and oxidative stress. VEGF, a cytokine that is upregulated early in diabetic retinopathy, has been shown to increase glucose transport in retinal endothelial cells (544). Additionally, hypoxia, which occurs at a later stage of diabetic retinopathy, also has been shown to upregulate glucose transport in retinal endothelial cells (565). GLUT1-induced glycosylation, possible phosphorylation, or conformational changes may also affect transport kinetics under hyperglycemic condition (141, 142, 155).
Interestingly, there are conflicting reports about GLUT1 expression levels in the retina under hyperglycemic conditions. In postpartum retinal tissues obtained from three long-term diabetic individuals with minimal or no clinical manifestation of retinopathy, a significant increase in GLUT1 expression was observed in the inner blood retinal barrier (BRB) (329). However, in 1-year-old Goto-Kakizaki (GK) rats that develop type-2 diabetes, no change in GLUT1 levels was observed (173). In another study, GLUT-1 levels were reduced in two weeks or two months of streptozotocin (STZ)-induced diabetic rats (38). These seemingly conflicting reports might be attributed to the use of different disease models, duration of diabetes, and analysis techniques.
In addition to GLUT1, other glucose transporters are expressed in various organs and tissues of the body. For example, GLUT2 is expressed in the gastrointestinal system, pancreatic islets, and the liver; GLUT3 is expressed in the central nervous system and the brain; and GLUT4 is expressed in adipose tissue and skeletal muscle (138). Of these latter three, GLUT3 has been reported to be expressed in retinal cells (609). Expression of GLUT3 remains constant or decreases in vitro under hyperglycemic conditions (314, 479), and remains constant in the retina of hyperglycemic rats (38).
Altered metabolism in the diabetic retina
There have been conflicting reports about oxygen consumption and arteriovenous oxygen differences in the retinas of diabetic rats (54, 128, 599), but with decreases of retinal oxygen consumption demonstrated in diabetic rabbits (272, 562) and cats (360). In all stages of retinopathy, arteriovenous oxygen differences in humans have been reported to decrease (230). The early decrease in retinal blood flow probably coincides with a decrease in oxygen consumption in patients with diabetes (95), which has been observed in several animal models. A blood flow reduction can result from a sustained or transient decrease in retinal oxygen consumption, which could be occurring in the diabetic retina as speculated by Small et al. (539) and Rimmer & Linsenmeier (490).
Models of retinal metabolic changes in diabetes often use rats or mice, and a difference in rat vs mouse metabolism was detailed in a study conducted by Obsorova et al. (436), where several metabolites such as lactate, ammonia, glutamate, free NAD+/NADH, and α-ketoglutarate were significantly decreased by 30-50% in STZ-induced diabetic rat retina, while only pyruvate and ammonia were decreased in the STZ-induced diabetic mouse retina.
A decrease in retinal metabolism under diabetic conditions could be explained by hyperglycemia-induced neuronal death. Increased retinal cell death has been observed in STZ-induced diabetic rats at 1-12 months of the disease (44). Additionally, in STZ-induced diabetic rats, photoreceptor apoptosis continues to increase in the time span of 4-24 weeks of hyperglycemia (453). The same phenomenon also is reported in STZ-induced diabetic mice (367, 380), and in Ins2Akita diabetic mice (367), where retinal neuronal cell death is observed. Moreover, retinal thinning, which is reported in diabetic rodents (380, 649) and humans (50, 63, 426, 586), is attributed in part to retinal cell death.
Altered oxygen levels in the diabetic retina
An uncontrolled growth of new blood vessels as a result of retinal hypoxia is thought to contribute to the development of diabetic retinopathy (31). There are several pieces of evidence that suggest a possible decrease in oxygen tension early in the diabetic retina; however, the evidence is not conclusive and additional studies may be required to resolve the time course of either hypoxia or hyperoxia early in the progression of experimental and human diabetes.
As will be discussed in more detail later in the review, retinal blood flow rates decrease early in the progression of diabetic retinopathy both in humans and in rodent models. Additionally, there has been a reported decrease in functional capillarity (RBC perfusion) of the retina of hyperglycemic rats following streptozotocin injection (progressively decreasing over a 15-90 day period), which can be partially blocked by inhibition of thromboxane synthesis or binding of thromboxane (126, 127). In addition to the changes in retinal capillary vasculature, various animal models of hyperglycemia result in non-perfused acellular capillaries (43, 299, 300, 655, 656). Hypoxia-inducible factor-1 and -2 (HIF-1 and HIF-2) expression and direct measurements of retinal oxygen tension, both of which are as discussed below, provide methods to examine potential alterations of oxygen tension in the diabetic retina.
HIFs are transcription factors that are upregulated when oxygen tension in the cell shifts from normoxia to hypoxia (296). HIF-1 contains two subunits, HIF-1α, which is expressed during times of normoxia but rapidly degraded, and HIF-1β, which is constitutively expressed (296). As oxygen tension decreases in the cell, HIF-1α becomes stabilized in the cytoplasm and forms a dimer with HIF-1β in the nucleus following translocation. A more comprehensive review of the regulation of HIF-1 can be found elsewhere (296). Stabilization of HIF-2α occurs at higher oxygen tensions than that of HIF-1α (457). HIF-1α regulates expression of glycolytic enzymes, while HIF-2α regulates expression of transforming growth factor-α (TGF-α) and erythropoietin (EPO), and both HIFs regulate VEGF and GLUT1 (457).
Oxygen levels in the retina have been measured using oxygen microelectrodes, and by exogenous bioreductive markers of hypoxia (e.g., pimonidazole). Microelectrode measurements have provided a direct measure of oxygen tension through the layers of the retina (644), while oxygen tension within a cell must decrease below 10 mmHg for bioreductive markers to bind macromolecules in the cell (363). This threshold for bioreductive sensors results in detection in the some parts of the retina even in control, non-diabetic eyes due to low oxygen tension levels that result from high oxygen utilization (645, 646).
Interestingly, within one year of inducing diabetes in cats and dogs, no signs of retinal hypoxia were reported in studies where microelectrodes have been used to measure oxygen concentration (162, 550, 551). Similar results have been reported by our lab in studies conducted in diabetic mice and rats, where using the hypoxia probe pimonidazole, or with hypoxia-inducible factor HIF-1α immunostaining, diabetes produced no significant signs of hypoxia in the early stages of hyperglycemia (1-6 months) (624, 625, 627), even with a substantial decrease in retinal blood flow rate (20-45%) (343, 344, 605, 606, 622, 626, 631). In contrast, an increase in retinal oxygen in the tissue was detected by our lab (using pimonidazole) at three months of hyperglycemia in diabetic rats (625), which was also reported at the same time point by Lau and Linsenmeier using oxygen microelectrodes (339). Nevertheless, increased pimonidazole and/or HIF-1α staining in the diabetic retina have been reported by other groups (124, 349, 370, 469, 483) at different time points, with de Gooyer et al. (124) reporting increased pimonidazole binding in the ganglion cell layer and inner nuclear layer of the mouse retina following five months of hyperglycemia. The increased binding of pimonidazole was eliminated in a hyperglycemic mouse model having outer retinal degeneration (124). In rats, there have been reports of increases in HIF-1α in the ganglion cell layer and inner nuclear layer two weeks after the induction of hyperglycemia (467), and in nuclear extracts eight days after induction of hyperglycemia (468). HIF-1α and HIF-2α in diabetic human retinas have not been found to differ from nondiabetic human retinas post mortem (198).
With longer-term diabetes, Linsenmeier et al. (360) found a significant increase in hypoxia in cats with 6-7 years of hyperglycemia using oxygen microelectrodes, with a significant reduction of almost 50% in inner retinal oxygen tension when compared to controls, and a decrease in photoreceptor oxygen utilization reported in one out of three diabetic cats.
Reactive oxygen species (ROS) in the diabetic retina
ROS production in the diabetic retina
Superoxide overproduction has been implicated in the development of diabetic retinopathy, where multiple enzymatic pathways have been shown to contribute to the production of the radical, such as by xanthine oxidase, mitochondrial complex Q, cyclooxygenase, p450 monooxygenase, NADPH oxidase, lipoxygenase, and uncoupled nitric oxide synthase. The increase in retinal superoxide levels and production have been reported in multiple studies of rodent models of diabetic retinopathy. Increased superoxide production, primarily from the mitochondria, and small amounts from NOS and NADPH oxidase, have been reported in STZ-diabetic rats with two months of hyperglycemia (150). Moreover, an increase in superoxide production in the photoreceptor layer, both in light- and dark-adapted STZ mouse diabetic eyes, was observed following two months of hyperglycemia (153).
Additionally, activity of superoxide dismutase (SOD), which converts superoxide into oxygen and hydrogen peroxide, was decreased in STZ-induced diabetic rats of both short-term hyperglycemia of 6 weeks (437) or 2 months (326), as well as with a longer-term duration of 11 months (325). However, it should be noted that an increase in SOD activity has been reported in the retinas of STZ-induced diabetic rats (136). The resulting production of hydrogen peroxide could be further converted into water with the help of catalase, or via its interaction with glutathione with the help of the enzyme glutathione peroxidase. In the diabetic rat retina, a moderate increase in catalase has been reported, with a possible decrease in glutathione peroxidase and no change in glutathione levels (436).
Role of ROS in the diabetic retina
Over the years, a “unified hypothesis” (67, 211, 427) has been developed in which superoxide overproduction plays a central role in diabetes-induced pathophysiological complications. In this hypothesis, superoxide initiates multiple damaging signaling pathways that occur concurrently, leading to the increased activity of the hexosamine pathway, the polyol pathway, the activation of PKC, and the increased formation of AGEs and their receptors. The increase in cellular glucose levels under hyperglycemia, the increase in pyruvate as a result of glucose metabolism, and participation of pyruvate in the Krebs cycle, all lead to an increased activity of the mitochondrial transport chain, and the overproduction of superoxide. This comes as a result of the production of the electron donors NADH and flavin adenine dinucleotide (FADH2) in the Krebs cycle, and their role in the electron transport chain. Proton extrusion increases, which causes the mitochondrial membrane voltage to reach and exceed its threshold, leading to the blockage of electron transport in complex III, and generation of superoxide by coenzyme Q donation of its electrons to O2. Additionally, the highly reactive hydroxide anion can be produced by the reduction of hydrogen peroxide that is formed by the conversion of superoxide by SOD. The highly reactive nitrogen species peroxynitrite (ONOO−) can be formed with the reaction of superoxide with nitric oxide, which can be damaging to the cell.
The initial hyperglycemia-induced superoxide production by the mitochondria may trigger the activation of additional pathways that could generate superoxide, such as uncoupled NOS or NADPH oxidase (211). The blocking of NADPH oxidase (NOX) activity in the diabetic mouse retina completely abolished ROS production, in a study in which mice were treated with the NOX inhibitor apocynin, or in NOX2-deficient mice. These results indicate a significant role for NADPH oxidase in the diabetes-induce ROS production in the diabetic retina. However, in findings reported by Nishikawa et al. (428), mitochondrial superoxide was shown to be the primary inducer of the hyperglycemia-induced retinal ROS production. The link between the two mechanisms could be explained by Al-Shabrawey et al. (19)., who suggested a role for mitochondria-derived ROS in activating PKC, which in turn could activate NOX, leading to sustained ROS production. Evidence for this connection was obtained in additional studies performed to show the ability of NOX to continue ROS production that was initiated by mitochondrial ROS (308, 345, 513).
The polyol pathway
The activation of the polyol pathway has detrimental and injurious effects on retinal cells. For example, there is an accumulation of the strongly hydrophilic, polyhydroxylated alcohol sorbitol, which initiates osmotic shock leading to hyperosmolar stress (310, 366, 568). Hyperosmolar stress leads to deleterious cellular responses including apoptosis, oxidative stress, DNA damage, inhibition of transcription and translation, mitochondrial adaptation, and cell cycle arrest (69). Consequently, hyperosmolar stress affects the retina and its functions due to the damage occurring to endothelial cells, loss of pericytes, thickening of the basement membrane, and injury to the retinal pigmented epithelium (165, 194).
Another metabolic product of polyol pathway activation, fructose, can induce cellular damage due to its high glycation activity, being a more potent glycating agent than glucose (560). Fructose is phosphorylated to fructose-3-phosphate, and further metabolized to 3-deoxyglucose, with both of these products potent glycating agents that can produce advanced glycation end-products (AGEs). AGEs can alter cellular components, leading to further cellular damage and impairment of the retina (293).
Studies have indicated a role for increased aldose reductase (AR) expression and sorbitol accumulation in disruption of the BRB. Increased AR levels in vascular retinal endothelial cells and Müller cells were found to be accompanied by a disruption of the BRB in the eyes of diabetic patients in a postmortem electron microscopic study (594). Additionally, NADPH is a cofactor for AR, with an increased activity of AR reducing the availability of NADPH to contribute to other important processes in the cell, such as the reduction of glutathione disulfide to glutathione (46). Moreover, the NADH/NAD+ ratio will increase due to the consumption of NAD+ by sorbitol dehydrogenase, leading to the initiation of pseudohypoxia, a state in which the tissue mimics a hypoxic response, in spite of normal oxygen levels, with an activation of HIF-1α (216, 614). Currently, multiple therapies for diabetic retinopathy are targeted toward the inhibition of AR; however, this strategy lacks complete specificity, with drug reactions with analogous AR enzymes, or with impaired AR detoxification and further injury (22).
Advanced glycation end products
AGEs are proteins and lipids that have been post-translationally modified and non-enzymatically glycated and oxidized by exposure to aldose sugars (516). AGEs can lead to vascular damage by modifying cytokines, hormones, and extracellular matrix proteins, leading to their dysfunction (65). Additionally, AGEs cross-link key molecules in the basement membrane, and can initiate deleterious signaling pathways that can alter multiple cellular functions leading to further damage (537).
Oxidative stress, protein turnover, and hyperglycemia are detrimental factors in the formation of AGEs. Schiff bases and Amadori products act as precursors to AGEs in the Maillard reaction. The reversible chemical reaction between the amino groups of proteins and glucose creates Schiff bases, which undergo rearrangement during continuous glucose exposure, and become the more stable Amadori products (528). Protein denaturation and crosslinking occur when the carbonyl groups, which are highly reactive and result from the reorganization of the Amadori products, react with amino, sulfhydryl, and guanidine functional groups of these proteins (10, 191). On the other hand, stable AGE compounds are created from the interaction of these carbonyl groups with lysine and arginine (364).
The receptors for advanced glycation end products (RAGEs), which belong to the immunoglobulin superfamily of receptors, interact with AGEs to initiate various signaling cascades within the cells leading to increases in nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) production, increases in VEGF production, inhibition of DNA synthesis, and an increase in retinal pericyte apoptosis (374), all of which can be detrimental to the cell. Moreover, studies have indicated a role of increased N-epsilon-carboxy methyl-lysine, an AGE product, in the development of type II diabetic complications in the retina (438). Currently, multiple techniques have been developed for the detection of AGE accumulation in the body, such as pulse wave analysis and skin autofluorescence. Additionally, various studies are being conducted with the hopes of finding successful therapies to limit AGE formation and its damage, such as the use of pigment epithelium derived factor (PEDF). PEDF has been shown to inhibit the formation of ROS due to AGEs, which in turn limits diabetes-induced pericyte dropout that can lead to BRB disruption (205, 530).
Protein kinase C (PKC) pathway activation
PKCs are a group of enzymes that are involved in various signaling pathways that can participate in multiple physiological and pathophysiological processes. PKCs belongs to the AGC kinase family that are widely distributed in various mammalian tissues, and include cAMP-dependent protein kinase A (PKA), cGMP-dependent protein kinase G (PKG), and lipid-activated PKC (327, 419). PKC activation, and the subsequent phosphorylation of target proteins at their serine and threonine residues (429), trigger a pathophysiological response that may contribute to the pathogenesis of diabetic retinopathy. Studies of this pathology have shown an increase in retinal vascular permeability, pericyte dropout, and retinal blood flow alterations that were associated with PKC activation (265, 452, 613).
The increased level of glucose in endothelial cells during hyperglycemia leads to the increased de novo synthesis of diacylglycerol (DAG), an endogenous activator of PKC. Glucose, which is transported into the endothelial cell via GLUT1, is metabolized via glycolysis to initially form the metabolic intermediate glyceraldehyde-3-phosphate, which is then converted via a multi-step process to DAG (629). DAG increases the affinity of PKC for Ca++, which results in increased PKC activity, and increased ROS and NO production (312). Additionally, enhanced PKC activity has been linked to an increase in VEGF production (480, 613) and the increase in TGF-β1 production that can contribute to the accumulation of extracellular matrix proteins (227, 628). Moreover, the overexpression of PKC under hyperglycemic conditions has been associated with an increase in retinal vascular permeability, which is possibly due to the modification of tight junction proteins and the cytoskeleton, leading to the disruption of the BRB (575).
Various isoforms of PKC have been shown to be expressed in the retina, with an increase in their activity under hyperglycemic conditions. PKC isoforms are derived from the alternative splicing of mRNA transcripts and multiple genes, have a wide variety of functions in biological systems (263), and can be divided into groups depending on their structure and cofactors. Classical or conventional PKCs (cPKCs) are activated by DAG, calcium, and phosphatidylserine (PS) or phorbol esters such as phorbol 12-myristate 13-acetate (PMA). These cPKCs include cPKC-α, -βI, -βII, and -γ (107, 402). Novel PKCs (nPKC) lack the characteristic C2 domain that is present in the classical PKCS. nPKCs are calcium-independent, but are activated by PS, PMA, and DAG, and include nPKC-δ, -ε, -θ and -η (419). In contrast, atypical PKCs (aPKC: aPKC-ı, -λ, and -ξ) differ from the two other classes of PKCs in that their regulatory domain has one zinc finger instead of two; however, aPKCs lack the C2 domain, as do nPKCs (400).
In the diabetic retina, studies have shown an increase in the activity of PKC-βI and βII relative to other PKCs (274, 531), with a possible role in hyperglycemic complications. PKC-α, -γ, and −δ activity also have been shown to increase under hyperglycemic conditions in the retina; however, this increase was not as substantial as with PKC-βI and βII (207). Additionally, hyperglycemia-induced retinal vascular leakage has been found to be attenuated with the inhibition of PKC-ξ, as are tight junction modifications due to VEGF and AGEs (545). The link between VEGF and the effects of hyperglycemia-induced PKC activation is further supported by the rapid activation and membrane relocation of PKC-α, PKC-γ, and PKC-δ with the intravitreal injection of VEGF, and the accompanying increase in retinal vascular permeability (12). These results indicate a possible role for hyperglycemia-induced PKC activation in the development and progression of diabetic retinopathy.
Inflammation
Damage to tissue and cells during injury due to hyperglycemia leads to the initiation of an inflammatory response, which involves the upregulation of inflammatory mediators and cytokines, and the activation and recruitment of leukocytes. This increase in the induction of inflammatory proteins, such as iNOS, cyclooxygenase-2 (COX-2), ICAM-1, VEGF, interleukin 1β, and tumor necrosis factor-α, occurs at the transcriptional level due to the activation of proinflammatory transcription factors, such as NF-κB (151, 567).
The recognition of diabetic retinopathy as an inflammatory disease started with the observation that diabetic patients with rheumatoid arthritis who were treated with salicylates had a lower incidence of retinopathy (474). The relationship between iNOS and diabetic retinopathy has been demonstrated by the decrease in leukocyte adhesion in the diabetic retinas of iNOS knockout mice, and in diabetic mice treated with the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME) (340). Moreover, diabetic patients have increased levels of neutrophils in the retina and choroid (386), and the levels of TNF-α and interferon-γ are elevated in the vitreous (597) and retina of diabetic patients (135).
Accumulation of platelets and macrophages in the retina also have been reported in experimental models of diabetes and in humans. In rats, platelet adhesion was found early in hyperglycemia; however, anti-platelet serum increased blood retinal barrier breakdown, which suggests that the platelets also play a protective role (635). Platelet aggregation is maintained in rats for up to nine months of hyperglycemia (277), and in humans with diabetes, platelet-fibrin thrombi have been observed in retinal capillaries (57). In addition, retinal tissue macrophages were found in hyperglycemic rats after two and nine months of hyperglycemia (521), and in the retinas of diabetic individuals with proliferative diabetic retinopathy (166). However, to our knowledge, the cause and effect relationship of platelets and macrophages to the development of diabetic retinopathy has not been established.
AGE formation has been found to play a role in the initiation of the inflammatory response in diabetic retinopathy. This response is possibly mediated via activation of the mitogen-activated protein kinase (MAPK) pathway due to AGE/RAGE interactions, and the resulting increases in retinal n3 polyunsaturated fatty acids (such as docosahexaenoic acid) (573) and n6 essential fatty acids (such as linoleic acid) (75). Monocyte chemoattractant protein-1 (MCP-1), VEGF, and ICAM-1 can become upregulated due to AGE/RAGE interactions, leading to increased leukocytosis and increased T-cell adhesion (273, 396).
In addition to the direct effects of hyperglycemia and AGE/RAGE interactions, hypoxia due to capillary dropout or blockage can play a role in the initiation of an inflammatory response in the diabetic retina. Hypoxia-induced TNF-α release from activated microglia and macrophages in the retina induces the expression of VEGF, MCP-1, and interleukin-8 in endothelial cells, which further enhances the inflammatory response in the diabetic retina (641).
Kallikrein-kinin system (KKS)
Multiple studies have indicated a possible role for the KKS in the development and progression of diabetic retinopathy. The KKS has important functions in the vascular system, where it mediates blood pressure regulation, blood flow, inflammation, and coagulation (49, 362, 461). The KKS can be divided into two distinct proteolytic pathways, plasma kallikrein and tissue kallikrein. Multiple tissues express tissue kallikrein, including the eye, while plasma kallikrein originates in the liver, and forms a complex with high molecular weight kininogen in the plasma upon its release (401). In patients with proliferative diabetic retinopathy, the levels and activity of PK in the vitreous is significantly higher than normal; however, tissue kallikrein has a low to undetectable level and activity in the vitreous of patients with diabetes (462). The two major G-protein cell surface receptors for the kinin family are the B1 and B2 receptors, with B2 constitutively expressed in the retina. While B1 is not expressed under physiologic conditions, its levels become elevated in diabetic and inflammatory conditions in the retina (3, 4).
In diabetic retinopathy, the activation of the KKS leads to a disruption of the BRB, alterations in vascular diameter and blood flow, inflammatory responses, and angiogenesis. Retinal vascular leakage is induced with the administration of recombinant plasma kallikrein intravitreally, or with B1 and B2 receptor agonists (258, 477). Additionally, intravitreal administration of bradykinin dilates retinal vessels and increases retinal blood flow (317, 542). This vascular effect also has been demonstrated in ex vivo experiments, where bradykinin was shown to dilate retinal vessels isolated from rat and porcine retinas, either non-pressurized in the presence of a preconstrictor to generate tone (3, 234), or pressurized without a preconstrictor (244). Activity of the kinin receptor B1, which is elevated in the diabetic retina, has been implicated in mediating the proinflammatory effects of the KKS, being associated with the increase in neutrophil recruitment and microglial activation (158, 267).
Diabetes-induced changes in retinal & choroidal blood flow
Alterations of blood flow in the diabetic retina
Retinal blood flow in individuals with diabetes has been reported to decrease by ~30-35% in the early stages of retinopathy (95), followed by a reversal back towards and even exceeding controls in more severe stages of the disease. The mechanisms responsible for the early decrease in flow could be important to discern, inasmuch as prevention of reduced blood flow and ischemia may attenuate the subsequent harmful angiogenesis. Therefore, retinal blood flow has been measured in various experimental animal models of diabetes, using a number of different techniques. As discussed in the following sections, most of the results from these studies are consistent with the early decrease seen in the human diabetic retina. However, the studies that are in contrast to this finding are of interest as well, and as discussed below, may be informative as to potentially deleterious structural changes occurring in the diabetic microvasculature.
Microsphere Technique
One method to measure blood flow in the retina (and in other tissues of the body) is via cardiac infusion of appropriate-sized microspheres that lodge in small precapillary arterioles throughout the body in proportion to the volumetric rate of blood perfusing the tissue. Following the optimization of this technique for the rat retina established by Wang et al. (601, 602), our lab has measured retinal blood flow in control vs diabetic STZ rats, at 8 weeks of hyperglycemia for the latter, finding an approximate 33% diabetes-induced decrease (347). This decrease is in contrast to the increase found in another STZ rat study in which a non-optimal number and a non-optimal size of microspheres were used (574). The microsphere technique has been employed in the retina in other animal studies of diabetes, finding a 40-45% decrease in blood flow in dogs following five months of hyperglycemia (539), and a similar 40-45% decrease in retinal blood flow following three weeks of hyperglycemia in rats (the latter not statistically significant) (219).
Intravital Microscopy
Retinal blood flow can be measured directly in anesthetized animals with the use of microscopy by measuring arteriolar (or venular) diameters and flow velocities (239). Diameter measurements are accomplished via a high molecular weight fluorescent plasma tracer with calibrated off-line video analysis, with velocities similarly measured from the distance traveled by fluorescently labeled microspheres (or red blood cells) in single video frames. Using this method, we have found significant diabetes-induced decreases in flow in Ins2(Akita) mice following six months of hyperglycemia (627), in nonobese diabetic (NOD) mice following three weeks of hyperglycemia (343), in STZ-injected rats after three weeks (but not after 1 week) of hyperglycemia (344), and in STZ-injected mice after 4 weeks (605, 622, 626, 631).
Laser Doppler
Using laser Doppler velocimetry, retinal blood flow has been found to decrease in diabetic humans without retinopathy (170, 222), and with background diabetic retinopathy (170). In another study using this technique, retinal blood flow measured in individuals with a mean diabetes duration of 12.5 years was decreased, while retinal blood flow was increased in diabetic individuals with a mean diabetes duration of 20 years (321). Patel et al. (458) reported in diabetic individuals with background retinopathy that retinal blood flow was increased, and that flow continued to increase as retinopathy advanced. Using laser Doppler velocimetry in a rat STZ model, we have found 35-40% decreases in flow compared with controls (347), although these measurements could be affected by other vascularized ocular tissues, such as the choroid.
Mean circulation time
Another method of measuring retinal blood flow utilizes the bolus infusion of a tracer, with the analysis of its retinal transit providing an index of flow. The infusion of a fluorescent plasma marker can be recorded via intravital video microscopy to analyze the entry of the tracer into and out of the retinal arterioles and venules, with the difference in mean transit times between the arterioles and venules being the mean circulation time (MCT). A flow index is then calculated from the retinal blood volume divided by the MCT, where the blood volume is assumed to be proportional to the average arteriolar diameters squared plus venular diameters squared. However, it should be mentioned that a limitation of using this blood volume estimation when making comparisons between groups is the assumption of similar capillary bed structures.
The MCT method has been used in rat models of diabetes to estimate retinal perfusion in the STZ model, with reported increases in MCT (but no changes in diameters) (71, 95, 96, 253, 259, 564) in the first two weeks of diabetes consistent with slower flow taking a longer time to traverse the retinal capillaries. After four weeks of diabetes, however, arteriolar diameters decreased with no change in mean circulation time (253). Subsequently, at eight weeks of diabetes, our lab found that the mean circulation time was drastically shortened compared to non-diabetic controls by ~67% (with no significant change in diameters), which would be consistent with retinal flow rates unexpectedly increasing dramatically by a factor of three (347). This result from our lab is in stark contrast to our results mentioned earlier using other techniques (microsphere infusion, microscopic measurements of diameters and velocities, laser doppler velocimetry), each of which indicate a significant decrease in flow (of ~30-40%), rather than a 300% increase. With the MCT technique being the only of these techniques suggesting an increase in retinal blood flow in the early weeks of STZ-induced diabetes in rats, we have hypothesized that shunting of blood through the superficial retinal layer, rather than traversing the intermediate (inner) and deep capillary layers, could result in a quick transit of blood through the retina that could be misinterpreted as faster flow (347). This shunting could possibly be the result of precapillary constriction (Figure 7) that would force more flow through the upstream vascular thoroughfares.
Figure 7. Transit pathways through the retinal circulation.
Normally, most retinal blood flow travels through the deep capillary layer. In diabetic rats, it is possible that a decreased proportion of flow goes through the deep layer, with rapid transit times possibly explained by flow preferentially perfusing the superficial and/or intermediate capillary layers due to vasoconstriction of the vessels (indicated by the asterisks) leading away from the superficial layer.
With the MCT technique dependent on capillary bed structure, another possibility for the rapid transit of dye through the STZ retina is a diabetes-induced loss of capillary density. However, the trypsin digest results obtained by Su et al. (558) have shown that capillary density in the rat model of STZ does not change over an approximate 2-year duration of diabetes. The trypsin digest model does not quantify the number of vessels actually being perfused; therefore, we have subsequently performed a study in which we quantified perfused vessel density in the STZ rat model (at the same time point in which MCT was greatly reduced), and found no change in the density of either the intermediate or deep capillary beds (347). Therefore, we speculate that shunting, due to vasoconstriction of the arterioles leading to the deep capillary beds, is a likely explanation for the shorter transit times in the STZ rat retina.
Alterations of blood flow in the diabetic choroid
Reports of changes in choroidal blood flow in diabetic individuals and in experimental models of hyperglycemia are inconsistent, and the mechanisms involved are not completely known. Choroidal circulation measured using pulsatile ocular blood flow has shown decreased flow prior to proliferative diabetic retinopathy, but with increased flow in proliferative diabetic retinopathy (210). Others have reported increased (372), decreased (333) or no change (517) in choroidal flow as severity of retinopathy increases in diabetic individuals. Savage et al. (510) reported increased choroidal blood flow in diabetic individuals with proliferative diabetic retinopathy, which decreased below the control group following panretinal photocoagulation. Choroidal flow was decreased in diabetic individuals with proliferative diabetic retinopathy when measured with laser Doppler flowmetry (519).
Alterations of vasoactive factors in diabetic retinopathy
Nitric Oxide
Diabetic retinopathy is associated with both increases as well as decreases in the production of NO. As discussed earlier, NO is produced from several different synthases (eNOS, iNOS, nNOS), which are not all affected the same in the diabetic retina. As will be discussed below, despite an overall increase in retinal NO in diabetes, evidence exists for a decrease in vascular NO.
With respect to the human retinal vasculature, Schmetterer et al. (515) have reported that intravenous L-NMMA administration in healthy individuals decreases ocular blood flow velocity, but to a lesser extent in type I diabetic patients, suggesting a possible pathophysiological role of NO signaling in diabetic retinopathy. In investigations using a porcine model of type I diabetes (243, 248), reductions were found in bradykinin-induced retinal arteriolar dilation, which also is attenuated by NOS inhibition (246, 248, 410). Even brief intraluminal exposure to high glucose in otherwise healthy porcine retinal arterioles suppresses bradykinin-induced dilations (248). Additionally, specific inhibitors of c-Jun N-terminal kinase (JNK) and JNK-interacting protein-1 (JIP1) fully restore the porcine retinal vascular response to bradykinin with both acute and chronic hyperglycemia; however, the protective effect of JNK and JIP1 inhibitors in the retinal vascular response is suppressed by NOS inhibition, indicating that JNK and JIP1 signaling pathways are involved in a glucose-dependent impairment of NO-mediated vascular function (248).
In in vitro cell cultures, retinal microvascular endothelial cells (RMECs) in high glucose conditions produce less NO (80, 103), with or without stimulation by acetylcholine (103). In hyperglycemia, the changes of endothelial NO levels both in vivo and in vitro have been shown to be controlled by expression and activation of eNOS. In vivo, mRNA expression of eNOS in the retina is reduced in the diabetic rat (652), and in vitro, incubation of RMECs in high glucose reduces eNOS protein expression (80, 103, 600). Several genomic data analyses have shown that polymorphisms of the eNOS gene are associated with diabetic retinopathy (37, 85, 93, 250, 371, 377, 394, 413, 508, 653). A deficiency of eNOS does not prevent the formation of the retinal vasculature during development; however, eNOS knockout mice develop more severe diabetic retinopathy (353).
The endothelium and NO-dependent functional hyperemia assay of retinal vascular dilation is attenuated in diabetes, in patients without (341) or with retinopathy (172, 201, 336, 356, 376, 420, 421, 460), with the vascular response continuing to decrease with increasing stages of diabetic retinopathy (172, 356, 376). This attenuated response in diabetes also occurs with acute hyperglycemia in healthy controls, with glucose levels temporarily increased to 300 mg/dL (148). The hyperglycemia-induced attenuation of flicker-stimulated functional hyperemia is also seen in a rat STZ model of type 1 diabetes (391, 392).
Despite the decreases in retinal vascular NO and eNOS in diabetes, the overall retinal and plasma levels vary. Some studies have reported decreased plasma levels of NO in diabetes (36, 113, 328, 652), whereas other studies have suggested the opposite (8, 35, 256, 279, 407, 447, 527). A study in diabetic STZ rats has shown retinal NO levels to be decreased with very high plasma glucose levels of 500-600 mg/dL, but increased at both mild (250-400 mg/dL) and moderate (400-500 mg/dL) levels (224). Increases in NO in the diabetic retina may be derived from either iNOS or nNOS as explained below.
Nitric oxide (152, 300, 324, 655) and retinal iNOS (5, 6, 151, 152, 340, 512, 577) have been reported to increase in human diabetes and in animal models of the disease. The increases in NO are inhibited by an iNOS inhibitor (152) and in iNOS-deficient mice (655). NO can bind to elevated levels of superoxide in the diabetic retina (300), with a resulting formation of peroxynitrite that can lead to PKC activation (475). In vitro, hyperglycemia increases NO production and iNOS expression in retinal Müller cells and in RMECs, with these increases (and cell death) suppressed by iNOS inhibition (151). The iNOS inhibitor aminoguanidine improves flicker-induced arteriolar dilation in diabetic rats (391), and as explained by these investigators, high levels of tissue NO might inhibit the glial release of dilatory agents, disrupting the connection between neuronal activity and vasodilation. It is possible that iNOS inhibition decreases retinal NO levels, allowing normal neurovascular coupling.
Increases in iNOS-dervied NO in the ganglion cell and inner plexiform layers of the retina (in STZ diabetic rats) do not activate soluble guanylate cyclase (512), which is able to dilate vascular smooth muscle. Instead, the elevated levels of NO produced by iNOS in the diabetic retina may induce the deleterious production of peroxynitrite, with this production attenuated by superoxide dismutase (512). Increased NO and overexpression of iNOS in insulin-dependent (76, 340) or -independent (77) diabetic rodents have been implicated in the breakdown of the blood-retinal barrier and development of diabetic retinopathy. Junctional barrier proteins in the diabetic mouse retina are protected by non-selective NOS inhibition and iNOS knockout (340).
In addition to increases in iNOS in diabetic retinopathy, nNOS expression and activity in the retina have been found to be increased in the inner nuclear layer and inner plexiform layer (215, 451). It is possible that increases in vascular-associated nNOS activity compensate for the eNOS deficiency in the retina and might be partially responsible for the increased NO levels in diabetic retinopathy.
Prostaglandins
Prostaglandin E (PGE2) and I2 (PGI2) have been found to be increased in STZ-injected rats following four weeks of hyperglycemia, with these increases attenuated by treatment with insulin (284). However, PGI2 levels have not been found to be elevated in the vitreous of diabetic individuals (456). It is unclear how the changes in PGI2 affect the retinal circulation during diabetes.
Thromboxane
The vasoconstrictor thromboxane A2 (measured as the stable metabolite thromboxane B2) has been reported to increase in platelets from diabetic rats (125-127). Inhibition of its synthesis by either a thromboxane synthase inhibitor (127), a combined thromboxane synthase inhibitor/receptor antagonist (126), or by aspirin (125), results in decreased TxA2 levels, and improved measures of retinal red blood cell perfusion through the capillary beds of diabetic STZ rats (126, 127). This improvement could be consistent with a role for the vasoconstrictor in the decreased flow seen in diabetic rodents. Indeed, retinal arterioles that are constricted in non-obese diabetic mice, and in STZ-injected mice and rats, are more dilated following administration of a thromboxane synthase inhibitor or a thromboxane receptor antagonist (343, 344, 622, 626). Additionally, expression of the thromboxane receptor and thromboxane synthase have been reported in mouse and rat retinas; however, there appear to be no differences in these expressions between control vs diabetic rodents following four weeks of hyperglycemia (623).
Adenosine
Adenosine infusion leads to retinal arteriolar vasodilation in euglycemic rats and in STZ-injected rats. This increase in arteriolar diameters is, in part, due to activation of NO synthase and to KATP channel opening (412). While the implications of the adenosine response are not fully understood in relation to physiological retinal circulation, increased activity of adenosine deaminase-2, an enzyme responsible for the breakdown of adenosine, has been reported in the vitreous of individuals with diabetes. Inhibition of the increased adenosine deaminase-2 activity, via miR-146b-3p, reversed the increased permeability and leukocyte adhesion in cultured human retinal endothelial cells (506).
Endothelin
Components of the endothelin system (i.e. endothelin and it receptors) are altered in ocular tissue of hyperglycemic rats (78, 79, 137) and in humans with proliferative retinopathy (439, 456); however, ET-1 levels are decreased in nonproliferative diabetic retinopathy (456). ETA receptor antagonism and inhibition of endothelin converting enzyme have been shown to attenuate decreases in retinal blood that occur in diabetic rats (563). Infusion of ET-3 initially increases retinal blood flow in control and diabetic rats through ET3/ETB binding; however, at later time points, ET-3 decreases retinal blood flow in control rats while flow remains elevated in diabetic rats, with flow-mediated changes found to occur via the ET-3/ETA receptor interaction (399). Endothelin is discussed more in depth elsewhere in a review of its role in diabetic retinopathy (547).
Angiotensin II
Although limited, there is evidence that angiotensin II plays a role in vascular abnormalities in the diabetic retina. It has been reported that angiotensin II receptor antagonism enhances flow and arteriolar diameters in NOD mice, with the latter changes specifically seen for arterioles in closer proximity to venules draining the retina (343). Administration of an angiotensin converting enzyme inhibitor improved diabetic retinal changes to tight junction loss, VEGF upregulation, and alterations in vascular permeability (307). Although encouraging, the clinical implications have not been confirmed (538).
Regulatory mechanisms of blood flow
Retinal blood flow regulation may be altered in diabetes, including via autoregulation and venular control of arteriolar diameters. Concerning autoregulation, this mechanism has been found to be impaired in the diabetic retinal circulation (154, 190, 535), with one study indicating that this deficiency is more prevalent in proliferative retinopathy (535). Additionally, diameters appear to be constricted in arterioles that are closely paired to venules in the retinas of diabetic rats and mice. The decrease in closely-paired arterioles is attenuated with inhibition of an angiotensin II receptor antagonist or a thromboxane synthase inhibitor (343, 344, 622).
Diabetes-induced changes in the retinal endothelial surface layer
Platelet endothelial cell adhesion molecule-1 (PECAM-1)
PECAM-1 is a 130 kDa transmembrane glycoprotein that belongs to the immunoglobulin G (IgG) superfamily of receptors with an immunoreceptor tyrosine-like inhibitory motif (ITIM) domain. It is highly expressed at the intercellular junction of endothelial cells (417), as well as on platelets, lymphocytes, and leukocytes. PECAM-1 has an integral role in cellular adhesion and transendothelial migration of leukocytes (417). In addition, PECAM-1 has been shown to have a role in apoptosis and cell signaling. For example of the latter, PECAM-1 modulates the functions of catenins, such as β and γ-catenin, by sequestering them to the plasma membrane and limiting their transcriptional activities (268, 269). Pathophysiological alterations to the retinal microvasculature in diabetic retinopathy involves increases in vascular permeability, leukocyte plugging of capillaries, altered cell signaling, and a decrease in cell survival (Figure 8), all of which could be related to PECAM-1 loss due to its important function in the endothelium. Thus, this section will cover in more detail the function of PECAM-1, and how its loss may contribute to the pathogenesis and progression of diabetic retinopathy.
Figure 8. PECAM-1 loss may contribute to the development of diabetic retinopathy.
Diabetic retinopathy is characterized by leukocyte plugging of capillaries, a decrease in endothelial cell survival, and an increase in vascular leakage. These phenomena also could occur with PECAM-1 loss, due to its important role in vascular endothelial cell functions.
Functions of PECAM-1
Since the discovery of PECAM-1, extensive studies have investigated the role of PECAM-1 in endothelial cells, and have led to the appreciation of the essential functions PECAM-1 has in the maintenance, survival, and integrity of the endothelium, in addition to its role in mediating the inflammatory response.
Role of PECAM-1 in inflammation
PECAM-1 can mediate inflammation by facilitating leukocyte transmigration and acting as a mechanosensor that can promote the production of NF-κB. On the other hand, PECAM-1 can prevent inflammation as a result of its role in cell survival and prevention of apoptosis. These conflicting roles of PECAM-1 are due to its physical and signaling properties that are mediated through its extracellular and cytoplasmic structures.
Role of PECAM-1 in leukocyte transmigration
The classic way by which PECAM-1 facilitates inflammatory responses is by its essential role in leukocyte transendothelial migration. PECAM-1 is the main component of the lateral border recycling compartment (616), which also contains CD99, JAM-A, CD155, IQ motif containing GTPase-activating protein-1, and vimentin (404, 561, 620). Leukocyte transmigration, by paracellular and intracellular pathways, involves the homophilic binding of endothelial PECAM-1 and leukocyte PECAM-1, which was demonstrated with the inhibition of leukocyte transmigration when the IgD1 homophilic binding site on the PECAM-1 extracellular domain was blocked (405). Additionally, when CD177 on neutrophils and/or IgD6 of PECAM-1 were blocked, neutrophil transmigration was inhibited, indicating an important role of PECAM-1 heterophilic binding (505). Moreover, not only is PECAM-1 involved in leukocyte transmigration via paracellular and intracellular pathways, but also in migration across the endothelial basement membrane (355).
Interestingly, PECAM-1 plays a more significant role in regulating the transmigration of neutrophils and monocytes than lymphocytes and eosinophils, due to a preferential selectivity to leukocyte sub-types (403). PECAM-1 also can regulate the rate and direction to leukocyte migration, which further demonstrates the complexity of the PECAM-1-mediated mechanism (621).
PECAM-1 involvement in leukocyte transmigration is dependent on the stimulus and the genetic background of the disease model. In studies using mice on a C57BL/6 genetic background, PECAM-1 was not required for leukocyte transmigration as it was not affected by PECAM-1 blockade (514). Additionally, PECAM-1 recruitment in leukocyte transmigration was initiated by specific stimuli, where IL-1β, L-NAME, and H2O2, but not TNF-α, led to leukocyte dependence on PECAM-1 for transmigration (621). Moreover, SHP-2 binding to the ITIM domain of the PECAM-1 cytoplasmic tail is not necessary for leukocyte transmigration. However, tyrosine 663, through a yet unknown mechanism, is required for this process (118).
Role of PECAM-1 as a mechanotransducer
Endothelial cells play an important role in sensing changes in blood shear stress, cyclic stretch, and osmolarity. Rearrangement of the endothelial cell cytoskeletal and modulation of vascular tone occurs with direct force applied to PECAM-1 using magnetic beads coated with a PECAM-1 antibody (442). These changes are sensed at the apical side of endothelial cells, and translated through signaling cascades to affect the endothelial cell cytoskeleton network and junctions, and also smooth muscle cells.
Tzima et al. observed a sensory complex that includes PECAM-1, vascular endothelial cadherin (VE-cadherin), and vascular endothelial growth factor receptor 2 (VEGFR2) (583). Tensile forces exert a mechanical pull on PECAM-1, leading to tyrosine phosphorylation on its cytoplasmic tail. This initiates a global endothelial cell response, including the activation of phosphatidylinositol 3-kinase and downstream effectors, and endothelial cell cytoskeleton rearrangement (102). Upon activation of PECAM-1, VE-cadherin acts as an adaptor to relay the change to VEGFR2, although some studies implicate a more significant role for VE-cadherin as a mechanosensor and not just as an adaptor protein (105). Upon VEGFR2 activation, a signaling cascade leads to the activation of phosphatidylinositol 3-kinase, and the subsequent activation of Akt and integrin, the upregulation of NF-κB, and the initiation of a proinflammatory response (104). In addition to its association with the mechanosensory complex, PECAM-1 is also in a complex with eNOS, and upon changes in shear stress, PECAM-1 rapidly dissociates from this complex prompting eNOS activation and NO production (182).
The importance of PECAM-1 as a mechanosensor is evident from studies conducted to understand the molecular mechanisms leading to atherosclerosis. Atherosclerotic lesions occur at areas of disturbed blood flow such as branch points of aortic arches. PECAM-1 senses these changes, and initiates the signaling cascade leading to lesion development (90). Moreover, PECAM-1 null mice have a decrease in lesion areas, further confirming the role of PECAM-1 as an atherogenic molecule (90).
PECAM-1 role in cell survival
Apoptosis, or programmed cell death, is an important aspect of the endothelial cell life cycle, which is marked by an intact plasma membrane, and the expression of apoptotic cell markers on the endothelial cell surface to target it for phagocytosis. However, if left unchecked, apoptosis can contribute to a multitude of pathophysiological complications including atherosclerosis, cancer, neurodegenerative disease, and diabetes-induced vascular damage. Additionally, endothelial cell apoptosis can lead to increased vascular permeability, exposure of thrombogenic basement membrane matrix, and increased platelet and leukocyte adhesion (617).
Noble et al. provided the first evidence in supporting a role for PECAM-1 in cell survival, where a decrease in endothelial cell apoptosis was observed with the homophilic binding of PECAM-1 on endothelial cells and monocytes following serum deprivation (430). PECAM-1 can mediate its anti-apoptotic properties through its ability to transduce signals that suppress apoptosis. Additionally, PECAM-1 was able to inhibit the release of cytochrome-c upon mitochondrial damage that was induced by cytotoxic factors activating the pro-apoptotic mitochondrial pore-forming Bcl-2-associated X protein (197). Moreover, sphinogosine-1-phosphate enhanced endothelial cell survival in a PECAM-1-dependent manner (357). Interestingly, blocking PECAM-1 homophilic binding or mutations in PECAM-1 immunoreceptor tyrosine-based inhibition motif domains blunted its ability to promote cell survival, indicating the importance of both the extracellular and cytoplasmic domains in mediating PECAM-1 cell survival functions (197).
The role of PECAM-1 in endothelial cell barrier function
One of the most critical functions of the vascular endothelium is to limit the access of blood and immune system components to the underlying tissue, which is even more critical in compromised tissue such as the brain and the retina. BRB breakdown directly contributes to the development of diabetic retinopathy, causing retinal tissue edema and promoting an inflammatory response. Therefore, the understanding of the mechanisms leading to BRB breakdown, and the molecular players contributing to it, may provide therapeutic targets for preventing and limiting development and progression of retinopathy in diabetes. Retinal endothelial cells form a tight layer that controls the passage of ions, proteins, and cells to the retinal tissue. This endothelial function is achieved by specialized cell adhesion molecules that maintain and preserve the endothelium barrier property. PECAM-1 is recognized as a cell adhesion molecule (20), that along with adherens junction proteins, tight junction proteins, and nectin constitute endothelial cell adhesion junction proteins (131).
Many studies have demonstrated the contribution of PECAM-1 to the maintenance of endothelial barrier function. In an experimental animal model of multiple sclerosis, PECAM-1 knockout mice exhibited an increased permeability of the blood-brain barrier (218). Additionally, CD44 knockout mice had a decreased level of PECAM-1 accompanied by an increase in vascular permeability that was returned to normal levels with increasing PECAM-1 levels (184). Moreover, PECAM-1-PECAM-1 homophilic interactions have been found to be required for the maintenance of vascular endothelial cell barrier function (476, 610). When mice are injected with antibodies against PECAM-1, vascular leakage from hepatic and renal blood vessels increases (177). Moreover, cultured PECAM-1-deficient endothelial cells have an increased permeability when exposed to histamine (218).
Role of PECAM-1 in facilitating functions of β-catenin
One of the possible mechanisms whereby PECAM-1 can contribute to barrier function is through its association with β-catenin, with PECAM-1 acting as a reservoir for phosphorylated β-catenin. Additionally, PECAM-1 facilitates dephosphorylation of β-catenin through the activation of Src homology region 2 domain-containing phosphatase-2 (SHP-2), which renders it active and able to associate with VE-cadherin to restore the endothelial barrier.
The first observation of the involvement of β-catenin in endothelial cell barrier function was made with the isolation of proteins associated with VE-cadherin, which found β-catenin linked with α-catenin and γ-catenin (446). VE-cadherin is a critical molecule in the maintenance and restoration of cell adhesion, and the association of β-catenin with VE-cadherin suggests a role in cell adhesion as well. VE-cadherin, which is a single pass transmembrane glycoprotein, associates with β-catenin at its cytoplasmic tail. Interestingly, the association of VE-cadherin with β-catenin is constitutive, and occurs early in the endoplasmic reticulum with the newly synthesized VE-cadherin (553). The β-catenin/VE-cadherin interaction contributes to the maintenance of the endothelial barrier, and limits its cytoplasmic pool and nuclear translocation, which is the site of its signaling functions.
The sequestration of phosphorylated and unphosphorylated β-catenin by PECAM-1 at the cell membrane protects β-catenin from being recognized at the cytoplasm by the destruction complex that is composed of Axin, anaphase-promoting complex, glycogen synthase kinase 3β, casein kinase 1, and protein phosphatase 2A (266). The latter three phosphorylate β-catenin, leading to its targeting for ubiquitination and subsequent degradation. This process is inhibited by the activation of the canonical Wnt signaling pathway, which after a series of molecular events, leads to the downstream cytoplasmic protein ‘Dishevelled’ forming a complex with the rate-limiting component of the destruction complex Axin, leading to its destabilization, and to the stabilization of β-catenin due to its decreased phosphorylation (51). The increase in cytosolic β-catenin can lead to translocation to the nucleus and the initiation of gene transcription. The mechanism by which β-catenin shuttles back and forth to the nucleus, in spite of its lacking a nuclear localizing signal or nuclear exporting signal, is not fully understood. However, there is evidence of a direct interaction between β-catenin and nuclear pore complex components (526, 532).
Possible mechanisms of PECAM-1 loss in the diabetic retina
Since its discovery, PECAM-1 has been shown to have many vital functions in the endothelium that surpass its initially recognized utility of being an endothelial cell marker. These functions assure the proper functioning and survival of endothelial cells, and preserves the vascular integrity through its role in endothelial barrier function. Thus, its loss can result in severe consequences, all of which can contribute to the development and progression of diabetic retinopathy.
Loss of PECAM-1 has been reported in the diabetic retina (74, 89), however, without an elaboration on the mechanism of this loss or its consequences. PECAM-1 can be cleaved by MMPs (409) such as MMP-2, MMP-9 (294), and MMP-7 (415), and its expression can be down-regulated by the cytokines TNF-α and IFN-γ (498) that play a significant role in mediating the inflammation associated with diabetes. MMPs and proinflammatory cytokines have been shown to increase in diabetes, but to our knowledge, no studies have been performed to link their activity with the diabetes-induced loss of PECAM-1 and the effect on retinal microvascular integrity.
Matrix metalloproteinases (MMPs)
MMPs play an important role in various physiological processes including extracellular matrix remodeling, wound healing, angiogenesis, and cell signaling (448). Additionally, MMPs can participate in pathophysiological processes, such as inflammation, cancer, arthritis, and cardiovascular disease (87, 204, 604). Under hyperglycemic conditions, MMP-2 and MMP-9 levels and activity are significantly upregulated. Several studies have indicated the role of MMP activation in hyperglycemia-induced ROS formation and oxidative stress leading to deleterious consequences in diabetes. In the diabetic retina, increased activation of MMP-2 and MMP-9 leads to mitochondrial dysfunction and the induction of apoptosis, which is possibly due to the translocation of MMP-9 to the mitochondria leading to a change in its redox state, since ROS can activate MMPs. MMPs residing in the mitochondria can become activated, leading to the degeneration of the mitochondrial membrane. Our lab has recently found evidence from both in vitro as well as in vivo studies implicating MMPs (especially MMP-2) in the hyperglycemia-induced loss of PECAM-1 from rat retinal microvascular endothelial cells (163).
One of the hallmarks of diabetic retinopathy is increased vascular leakage due to BRB breakdown, which can be facilitated through the degradation of cell adhesion molecules such as PECAM-1, which has been shown to be cleaved by MMPs. Various cleavage sites by MMPs have been found on PECAM-1 using in silico analysis, indicating the possible role MMPs can play in decreasing PECAM-1 levels under hyperglycemic conditions. These results indicate the multifaceted role by which MMPs can contribute to the progression of diabetic retinopathy, where they can function as proinflammatory and proapoptotic factors, and by cleaving and remodeling the endothelial cell surface and cell adhesion proteins.
The proinflammatory cytokine tumor necrosis factor-α (TNF-α)
Inflammation and proinflammatory factors play a key role in the progression of diabetic retinopathy. One of the most potent proinflammatory mediators is TNF-α, which is a key inflammatory cytokine that is produced by many cells, including endothelial cells, macrophages, lymphocytes, and mast cells (373). TNF-α is initially synthesized and produced as a type II transmembrane protein and cleaved by TNF-α converting enzyme producing soluble TNF-α (260). Both transmembrane and soluble TNF-α exert their effects on endothelial cells by interacting with their receptors, TNFR1 and TNFR2, leading to increased vascular leakage, and the expression of proinflammatory molecules that further worsens the inflammatory response (598).
Serum TNF-α levels are significantly increased in patients with type I diabetes (478), which is indicative of it role in promoting the inflammatory state in diabetes. Several studies have investigated the effect of TNF-α on cell adhesion molecules such as PECAM-1 due to its important role in endothelial barrier function. TNF-α decreases PECAM-1 levels (290, 498, 501, 511, 529, 554), which can influence leukocyte transmigration and vascular leakage. These results indicate a possible role for TNF-α in the hyperglycemia-induced PECAM-1 loss in the diabetic retina, possibly leading to development and progression of diabetic retinopathy using a yet not fully understood mechanism.
Diabetes-induced changes in the retinal endothelial glycocalyx
Structure and functions of the endothelial glycocalyx
The endothelial glycocalyx is a dynamic carbohydrate-rich layer on the cell surface, with the general structure depicted in Figure 9. The glycocalyx is attached to the endothelium via its backbone molecules, mainly proteoglycans, which consist of a core protein and unbranched glycosaminoglycan chains (GAGs) covalently attached to the core protein. The membrane-spanning syndecans and glycosyl-phosphatidylinositol anchored glypicans are the primary core proteins. There are five main types of GAGs: heparan sulfate, hyaluronic acid, chondroitin sulfate, dermatan sulfate, and keratan sulfate (27, 319, 486). However, hyaluronic acid, instead of binding to core proteoglycans, binds to its receptors CD44 and the receptor for hyaluronan mediated motility (319). Additional points of attachments to the endothelium are provided by other backbone glycoproteins, such as selectins, integrins and immunoglobulins (27, 319, 486). Proteoglycans and glycoproteins form a network with both plasma and endothelium-derived soluble molecules such as albumin and orosomucoid, forming a mesh-like structure on the endothelium (27, 319, 486). The glycocalyx inhibits leukocyte and platelet adhesion to the endothelium (27, 319, 486), and also regulates the distribution of red blood cells in microvessels (72). In addition, the glycocalyx acts as a mechanosensor, regulates vascular permeability, and is a reservoir of antithrombotic factors including anti-thrombin III (27, 319, 486). As described in the following sections, hyperglycemia and diabetes can result in a loss of the glycocalyx, which could have an effect on these functions. In many ways, this mesh-like structure helps maintain the physiological and quiescent environment of endothelial cells.
Figure 9. Glycocalyx components.
The structure of the endothelial glycocalyx on the plasma side of the cell includes proteoglycans (such as syndecans and glypican-1) and glycosaminoglycans (such as heparan sulfate, chondroitin sulfate, and hyaluronic acid).
In vitro loss of glycocalyx in hyperglycemia
In an in vitro glomerular endothelial cell (GEnC) model of hyperglycemia, Singh et al. (536) observed a reduced expression of heparan sulfate in the GEnCs when treated with high glucose (25.5 mM) compared to normal glucose (5.5 mM). However, there was no change in the expression of proteoglycans (syndecan-1, syndecan-4, glypican-1, versican-1, or perlecan-1). In the same study, high glucose treatment also led to a significant increase in the passage of albumin across GEnC monolayers despite no difference in the expression of VE-cadherin, a major component of the interendothelial junction (536), with increased endothelial permeability one potential consequence of a diabetes-induced loss of the vascular glycocalyx.
Heparan sulfate helps comprise flow-sensing mechanisms on the surface of endothelial cells, with this mechanism impaired by both heparinase treatment as well as hyperglycemia in human aortic endothelial cells (64). A similar hyperglycemia-induced impairment of glycocalyx-mediated mechanotransduction has been reported for bovine aortic endothelial cells, with a loss of heparan sulfate coincident with shear-dependent eNOS activation and control of hydraulic conductivity (365). Hyperglycemia and TNF-α have been shown to have an additive effect on the shedding of the endothelial glycocalyx of human umbilical vein endothelial cells (HUVECs). TNF-α (50 ng/ml) and glucose (200 mg/dL), when applied individually to HUVECs, led to similar (22-27%) reductions in the glycocalyx thickness and shedding of syndecan-1 and hyaluronic acid (144). However, when administered together, there was 42% decrease in the glycocalyx thickness, a 4-fold increase in shedding of syndecan-1, and a 10-fold increase in shedding of hyaluronic acid.
Loss of glycocalyx in diabetes
Degradation of the systemic glycocalyx has been noted in subjects with diabetes as well as in animal models of the disease. In human studies, patients with type 1 diabetes were found to have a smaller microvascular glycocalyx in sublingual capillaries compared to controls (424, 434). This decrease was accompanied by increases in plasma hyaluronic acid and hyaluronidase under hyperglycemia (423, 424) that were even greater in patients with microalbuminuria. Another clinical study showed a reduction in systemic glycocalyx volume (and increased plasma level of hyaluronic acid) upon application of a hyperglycemic clamp in healthy males, which was reversed with N-acetylcysteine treatment (425). The glycocalyx excludes plasma molecules, in part based on size, with 70-kD dextrans excluded from the glycocalyx substantially more than 40-kD dextrans. In a db/db type 2 diabetes mouse model, investigators determined that hyperglycemia diminishes the systemic glycocalyx exclusion of 70-kD dextran, compared to normoglycemic controls (164), with the glycocalyx barrier improved with metformin treatment. However, it should be mentioned that a limitation of the measurement of systemic glycocalyx volume is that the lower molecular weight 40-kD dextran can more easily pass through the endothelium into the tissue, introducing an error in the calculation (389).
The glycocalyx is likely to be affected in many tissues throughout the body in diabetes, and has been studied not only at a systemic level, but also in specific tissues, for example, the kidneys and retina that are sites of microvascular injury in diabetes. In a db/db mouse model of type-2 diabetes, a study of kidney glomeruli found a reduction of sulfation of the glycocalyx GAG chondroitin sulfate (484). In the same study, disaccharide analysis of chondroitin sulfate showed reductions in 4-O and 6-O sulfated disaccharides, with an increase in non-sulfated disaccharide residues. The study also found a significant decrease in the mRNA expression of chondroitin 4-O sulfotransferase in the diabetic kidney.
In diabetic nephropathy, a decrease in heparan sulfate (which is found on the endothelial surface and basement membrane of the glomerular capillaries) has been observed, which has shown to correlate inversely with the level of urinary protein excretion in diabetic patients (203). The same report demonstrated decreased albuminuria and improved renal function when diabetic mice were treated with a heparanase inhibitor (203). Additionally, the loss of heparan sulfate from the glomeruli of type 2 diabetes and overt diabetic nephropathy patients is correlated with an increase in heparanase (585). These studies suggest that heparan sulfate plays an important role in glomerular protein passage, and its loss is associated with the development of proteinuria and diabetic nephropathy.
Loss of glycocalyx in the diabetic retina
The structure of the glycocalyx may differ from tissue to tissue, and as of yet, the components of the retinal glycocalyx are yet to be established. However, a few initial studies have indicated that the retinal glycocalyx is lost (by ~35-50%) in diabetic humans, rats, and mice. In humans, an in vivo clinical study observed a 50% loss of the retinal glycocalyx in type 2 diabetes patients, which was partially restored with sulodexide, a highly purified combination of heparan sulfate and dermatan sulfate (62). In STZ-induced diabetes in rats, a reduction in the retinal glycocalyx thickness was observed ex vivo in tissue sections using transmission electron microscopy (330). In a another study, which was from our lab using a genetic Ins2(Akita) model of type 1 diabetes in mice, we observed an approximate 35% reduction in the glycocalyx thickness of retinal arterioles in vivo, but not in venules (346).
Mechanisms of glycocalyx degradation
The mechanisms underlying the diabetes-induced degradation of the endothelial glycocalyx are poorly understood. Hyperglycemia is thought to not only decrease the synthesis of glycocalyx components (mainly via production and/or sulfation of GAG chains) (100, 484, 595), but also increase the rate of glycocalyx shedding. Glycosaminoglycans and proteoglycans in the glycocalyx can be cleaved by matrix metalloproteinases (MMPs) (160, 361, 485), heparan sulfate can be cleaved by heparinase (533), and hyaluronic acid can be cleaved by hyaluronidase (548). Increased concentrations of MMPs have been found in retinal tissue of diabetic rats, and also in rat retinal microvascular endothelial cells when treated with high glucose (25mM) (163). Additionally, plasma and vitreous samples from diabetes patients have shown an elevation of MMPs (42, 289, 431). Heparanase levels are elevated in plasma and urine samples of type 2 diabetes patients (525), in whom plasma heparanase is positively correlated with blood glucose levels in the early stages of diabetic nephropathy (654). Diabetes patients also exhibit higher plasma hyaluronidase levels (423, 424), although our lab did not find an increase in plasma hyaluronidase activity in type 1 diabetic Akita mice compared to controls (346). However, hyaluronidase deficiency in STZ-diabetic mice has been shown to preserve the glycocalyx structure in diabetes (146).
Besides MMPs and GAG-specific enzymes, other mediators have been implicated as playing a role in the degradation of the glycocalyx in diabetes. For example, reactive oxygen species have been shown to mediate the mechanism by which hyperglycemia enhances the shedding of the glycocalyx following trauma/shock (143). Toll-like receptor 2 and 4 (TLR2 and TLR4) also have been speculated to mediate hyperglycemia-induced changes in the glycocalyx. Hyperglycemia upregulates mRNA and protein expression of TLR2 and TLR4, reduces heparan sulfate expression on human macrovascular aortic endothelial cells, and increases endothelial release of hyaluronic acid (449). These effects were reversed when TLR2 and TLR4 signaling was blocked using inhibitory peptides and siRNA knockdown (449).
Role of the glycocalyx in RBC distribution
Several studies indicate that one of the many functions of the glycocalyx is the regulation of RBC distribution through a microvascular bed, with consequences for local hematocrit (238). RBCs are excluded from the endothelial glycocalyx in microvessels (593), which reduces the microhematocrit, and correspondingly, when the endothelial glycocalyx is degraded with either hyaluronidase (72), heparinase (139), or light-dye treatment (593), capillary hematocrit increases by 60-100%.
Not only does the glycocalyx help control microhematocrit, but it also appears to promote the homogeneous distribution of those RBCs in the various capillaries of a bed. This finding has been predicted via mathematical models in which apparent viscosity plays a role in this mechanism (383). Results from in vivo experiments agree with the mathematical models, inasmuch as the heterogeneity of capillary perfusion substantially increases following degradation of the glycocalyx with hyaluronidase (72), causing some capillaries to stop flow, and other capillaries to be perfused solely by plasma (no RBCs). A consequence of increased heterogeneity of RBC distribution through a capillary bed is a decrease in the efficiency of oxygen transport to the surrounding tissue (282, 292, 444). Given the loss of glycocalyx in diabetes, it can be speculated that RBC distribution will be affected, which may contribute to the zones of ischemia that are observed in the human retina.
Conclusion
The retina is particularly vulnerable to the deleterious effects of continued hyperglycemia in the diabetic state. Diabetes-induced changes to the retinal circulation warrant the classification of the ensuing retinopathy as a microvascular complication of the disease. It is well-known that the proliferative phase of diabetic retinopathy is associated with uncontrolled angiogenesis that can interfere with vision, but considerable evidence has been collected showing significant vascular changes occurring earlier in the disease prior to the vascular proliferation.
Among the early changes in the diabetic retina are decreases in perfusion and a possible diversion of flow away from the deep capillary bed that feeds important retinal neurons. Evidence in the literature implicates several vasoactive pathways, such as thromboxane A2, endothelin-1, angiotensin II, and decreases in endothelial nitric oxide, in the altered perfusion. Additionally, recent studies have demonstrated significant changes in the endothelial surface layer (including a loss of platelet endothelial cell adhesion molecule-1) and the glycocalyx, with these molecules and structures serving vital functions in a healthy vasculature. Future studies on the prevention of the later stages of diabetic retinopathy should focus in part on preventing these early changes in the retinal microcirculation.
DIDACTIC SYNOPSIS.
Major teaching points:
The retina includes layers of several types of neurons that receive oxygen and nutrients from two different vascular beds, i.e., the retinal circulation and the choroidal circulation.
The retina and its circulation are particularly vulnerable to the deleterious effects of continued hyperglycemia in the diabetic state.
Diabetes-induced changes to the retinal circulation warrant the classification of the ensuing retinopathy as a “microvascular complication” of the disease.
Among the early changes in the diabetic retina are decreases in perfusion and a possible diversion of flow away from the deep capillary bed that feeds important retinal neurons.
Recent studies have demonstrated significant changes in the endothelial surface layer (including a loss of platelet endothelial cell adhesion molecule-1) and the glycocalyx, with these molecules and structures serving vital functions in a healthy vasculature.
Acknowledgement:
Supported by funding from the National Institutes of Health (NIH) EY025632.
DIDACTIC LEGENDS
Figure 1. Teaching points: Diabetes is associated with complications of larger vessels in the body. These macrovascular complications include peripheral artery disease, cardiovascular disease, and stroke. Diabetes also is associated with microvascular complications that especially affect the retina (diabetic retinopathy), nerves (diabetic neuropathy), and the kidneys (diabetic nephropathy).
Figure 2. Teaching points: Ocular tissue can be divided into three layers: 1) an outer layer consisting of the sclera and the cornea (also known as the fibrous coat), 2) an intermediate layer consisting of the iris, the ciliary body, and the choroid (the vascular coat), and 3) an internal layer consisting of the retina (the nervous coat).
Figure 3. Teaching points: The retina contains several different types of cells that are segregated into layers. The layers and membranes of the retina starting from the outermost to the innermost layer are the 1) retinal pigmented epithelium (RPE), 2) photoreceptors (PR), 3) outer limiting membrane (OLM), 4) ) outer nuclear layer (ONL), 5) outer plexiform layer (OPL), 6) inner nuclear layer (INL), 7) inner plexiform layer (IPL), 8), ganglion cell layer (GCL), 9) nerve fiber layer (NFL), and 10) inner limiting membrane (ILM).
Figure 4. Teaching points: The retina is a highly metabolic tissue that requires sufficient oxygen for proper function. The inner half of the retina receives oxygen from the retinal circulation, while the outer half receives oxygen from the choroidal circulation.
Figure 5. Teaching points: Ocular blood supply derives from the internal carotid artery that branches off of the aorta. The internal carotid artery then branches into the ophthalmic artery that leads to the eye. The blood vessels supplying blood to the inner and outer retina, the iris, and the ciliary body originate from the ophthalmic artery. The ophthalmic artery further divides to give rise to the anterior ciliary arteries, the long and short posterior ciliary arteries, and the central retinal artery.
Figure 6. Teaching points: The retinal arteries branch off of the central retinal artery into a superficial layer that is located in the ganglion cell layer. These arterioles branch into an intermediate capillary bed located in front of the inner nuclear layer, and then into the deep capillary bed located behind the inner nuclear layer.
Figure 7. Teaching points: Retinal blood flow can take several different pathways through the tissue, through either the superficial, intermediate, or deep capillary beds. Normally, most retinal blood flow travels through the deep capillary layer. In diabetic rats, the transit time through the retina is exceedingly fast despite slower velocity: therefore, it is possible that a decreased proportion of flow in the diabetic rat goes through the deep layer, with rapid transit times possibly explained by flow preferentially perfusing the shorter superficial and/or intermediate capillary pathways due to vasoconstriction at sites indicated by the asterisks.
Figure 8. Teaching points: Diabetic retinopathy is characterized by leukocyte plugging of capillaries, a decrease in endothelial cell survival, and an increase in vascular leakage. These phenomena also could occur with the loss of platelet endothelial cell adhesion molecule-1 (PECAM-1), due to its important role in these same related functions.
Figure 9. Teaching points: Endothelial cells (as well as other cell types) have a surface layer that consists of molecules having sugar residues. This “sugar-coat”, or glycocalyx, serves many functions. The structure of the endothelial glycocalyx on the plasma side of the cell includes proteoglycans (such as syndecans and glypican-1) and glycosaminoglycans (such as heparan sulfate, chondroitin sulfate, and hyaluronic acid).
REFERENCES
- 1.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853, 1998. [PubMed] [Google Scholar]
- 2.Retinal Conditions - Common Eye Disorders∣Vision Health Initiative (VHI)∣. http://www.cdc.gov/visionhealth/basic_information/eye_disorders_retinal.htm: The Vision Health Initiative (VHI), 2014. [Google Scholar]
- 3.Abdouh M, Khanjari A, Abdelazziz N, Ongali B, Couture R, Hasséssian HM. Early upregulation of kinin B1 receptors in retinal microvessels of the streptozotocin-diabetic rat. British journal of pharmacology 140: 33–40, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdouh M, Talbot S, Couture R, Hassessian HM. Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B(1) and B(2) receptors. British journal of pharmacology 154: 136–143, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu El-Asrar AM, Desmet S, Meersschaert A, Dralands L, Missotten L, Geboes K. Expression of the inducible isoform of nitric oxide synthase in the retinas of human subjects with diabetes mellitus. American journal of ophthalmology 132: 551–556, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Abu El-Asrar AM, Meersschaert A, Dralands L, Missotten L, Geboes K. Inducible nitric oxide synthase and vascular endothelial growth factor are colocalized in the retinas of human subjects with diabetes. Eye (London, England) 18: 306–313, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. American journal of ophthalmology 118: 445–450, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Adela R, Nethi SK, Bagul PK, Barui AK, Mattapally S, Kuncha M, Patra CR, Reddy PN, Banerjee SK. Hyperglycaemia enhances nitric oxide production in diabetes: a study from South Indian patients. PLoS One 10: e0125270, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agardh CD, Eckert B and Agardh E. Irreversible progression of severe retinopathy in young type I insulin-dependent diabetes mellitus patients after improved metabolic control. J Diabetes Complications 6: 96–100, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed MU, Thorpe SR and Baynes JW. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. The Journal of biological chemistry 261: 4889–4894, 1986. [PubMed] [Google Scholar]
- 11.Ahnelt PK and Kolb H. The mammalian photoreceptor mosaic-adaptive design. Prog Retin Eye Res 19: 711–777, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes 46: 1473–1480, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R. Diabetic retinopathy. Diabetes Care 21: 143–156, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2: a006429, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 100: 174–190, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Akiba J, Arzabe CW and Trempe CL. Posterior Vitreous Detachment and Neovascularization in Diabetic Retinopathy. Ophthalmology 97: 889–891, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Al-Shabrawey M, El-Remessy A, Gu X, Brooks SS, Hamed MS, Huang P, Caldwell RB. Normal vascular development in mice deficient in endothelial NO synthase: possible role of neuronal NO synthase. Molecular vision 9: 549–558, 2003. [PubMed] [Google Scholar]
- 19.Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci 49: 3239–3244, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol 114: 1059–1068, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alder VA, Su EN, Yu DY, Cringle SJ, Yu PK. Asymmetrical response of the intraluminal and extraluminal surfaces of the porcine retinal artery to exogenous adenosine. Experimental eye research 63: 557–564, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Alexiou P, Pegklidou K, Chatzopoulou M, Nicolaou I, Demopoulos VJ. Aldose reductase enzyme and its implication to major health problems of the 21(st) century. Curr Med Chem 16: 734–752, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Alm A The effect of sympathetic stimulation on blood flow through t,e uvea, retina and optic nerve in monkeys (Macacca irus). Experimental eye research 25: 19–24, 1977. [DOI] [PubMed] [Google Scholar]
- 24.Alm A and Bill A. Blood flow and oxygen extraction in the cat uvea at normal and high intraocular pressures. Acta physiologica Scandinavica 80: 19–28, 1970. [DOI] [PubMed] [Google Scholar]
- 25.Alm A and Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Experimental eye research 15: 15–29, 1973. [DOI] [PubMed] [Google Scholar]
- 26.Alm A and Bill A. The oxygen supply to the retina. II. Effects of high intraocular pressure and of increased arterial carbon dioxide tension on uveal and retinal blood flow in cats. A study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Acta physiologica Scandinavica 84: 306–319, 1972. [DOI] [PubMed] [Google Scholar]
- 27.Alphonsus CS and Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 69: 777–784, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Ames A 3rd, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci 12: 840–853, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE, Abrams GW. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 38: 36–47, 1997. [PubMed] [Google Scholar]
- 30.Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 47: 1953–1959, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Arden GB and Sivaprasad S. The pathogenesis of early retinal changes of diabetic retinopathy. Doc Ophthalmol 124: 15–26, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Artwohl M, Brunmair B, Furnsinn C, Holzenbein T, Rainer G, Freudenthaler A, Porod EM, Huttary N, Baumgartner-Parzer SM. Insulin does not regulate glucose transport and metabolism in human endothelium. Eur J Clin Invest 37: 643–650, 2007. [DOI] [PubMed] [Google Scholar]
- 33.ASHTON N and HARRY J. THE PATHOLOGY OF COTTON WOOL SPOTS AND CYTOID BODIES IN HYPERTENSIVE RETINOPATHY AND OTHER DISEASES. Trans Ophthalmol Soc U K 83: 91–114, 1963. [PubMed] [Google Scholar]
- 34.Astin M, Stjernschantz J and Selen G. Role of nitric oxide in PGF2 alpha-induced ocular hyperemia. Experimental eye research 59: 401–407, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Aydın A, Orhan H, Sayal A, Özata M, Şahin G, Işımer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clinical biochemistry 34: 65–70, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Ayub T, Khan SN, Ayub SG, Dar R, Andrabi KI. Reduced nitrate level in individuals with hypertension and diabetes. Journal of cardiovascular disease research 2: 172–176, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azmy R, Dawood A, Kilany A, El-Ghobashy Y, Ellakwa AF, El-Daly M. Association analysis of genetic variations of eNOS and alpha2beta1 integrin genes with type 2 diabetic retinopathy. Appl Clin Genet 5: 55–65, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badr GA, Tang J, Ismail-Beigi F, Kern TS. Diabetes downregulates GLUT1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes 49: 1016–1021, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Bae SW, Kim HS, Cha YN, Park YS, Jo SA, Jo I. Rapid increase in endothelial nitric oxide production by bradykinin is mediated by protein kinase A signaling pathway. Biochemical and biophysical research communications 306: 981–987, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Balasubramanian R and Gan L. Development of Retinal Amacrine Cells and Their Dendritic Stratification. Curr Ophthalmol Rep 2: 100–106, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballantyne AJ. THE OCULAR MANIFESTATIONS OF SPONTANEOUS SUBARACHNOID HAEMORRHAGE. Br J Ophthalmol 27: 383–414, 1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ban CR, Twigg SM, Franjic B, Brooks BA, Celermajer D, Yue DK, McLennan SV. Serum MMP-7 is increased in diabetic renal disease and diabetic diastolic dysfunction. Diabetes Res Clin Pract 87: 335–341, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci 46: 2210–2218, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102: 783–791, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barcsay G, Seres A and Nemeth J. The diameters of the human retinal branch vessels do not change in darkness. Invest Ophthalmol Vis Sci 44: 3115–3118, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Barnett PA, Gonzalez RG, Chylack LT Jr., Cheng HM. The effect of oxidation on sorbitol pathway kinetics. Diabetes 35: 426–432, 1986. [DOI] [PubMed] [Google Scholar]
- 47.Beltramo E and Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem 20: 3218–3225, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Benedito S, Prieto D, Nielsen PJ, Nyborg NC. Role of the endothelium in acetylcholine-induced relaxation and spontaneous tone of bovine isolated retinal small arteries. Experimental eye research 52: 575–579, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Bhat M, Pouliot M, Couture R, Vaucher E. The kallikrein-kinin system in diabetic retinopathy. Prog Drug Res 69: 111–143, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Biallosterski C, van Velthoven ME, Michels RP, Schlingemann RO, DeVries JH, Verbraak FD. Decreased optical coherence tomography-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br J Ophthalmol 91: 1135–1138, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316: 1619–1622, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Bill A Autonomic nervous control of uveal blood flow. Acta physiologica Scandinavica 56: 70–81, 1962. [DOI] [PubMed] [Google Scholar]
- 53.Bill A and Sperber GO. Control of retinal and choroidal blood flow. Eye (London, England) 4 ( Pt 2): 319–325, 1990. [DOI] [PubMed] [Google Scholar]
- 54.Blair NP, Wanek JM, Mori M, Shahidi M. Abnormal retinal vascular oxygen tension response to light flicker in diabetic rats. Invest Ophthalmol Vis Sci 50: 5444–5448, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blaschuk OW, Oshima T, Gour BJ, Symonds JM, Park JH, Kevil CG, Trocha SD, Michaud S, Okayama N, Elrod JW, Alexander JS, Sasaki M. Identification of an occludin cell adhesion recognition sequence. Inflammation 26: 193–198, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Bleicher SJ, Lee TY, Bernstein R, Schachter L, Temes R, Krupin T, Santiago J, Becker B, Waltman SR. Effect of blood glucose control on retinal vascular permeability in insulin-dependent diabetes mellitus. Diabetes Care 3: 184–186, 1980. [DOI] [PubMed] [Google Scholar]
- 57.Boeri D, Maiello M and Lorenzi M. Increased prevalence of microthromboses in retinal capillaries of diabetic individuals. Diabetes 50: 1432–1439, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Bolinger MT and Antonetti DA. Moving Past Anti-VEGF: Novel Therapies for Treating Diabetic Retinopathy. International journal of molecular sciences 17: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol 82: 561–568, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braunagel SC, Xiao JG and Chiou GC. The potential role of adenosine in regulating blood flow in the eye. Journal of ocular pharmacology 4: 61–73, 1988. [DOI] [PubMed] [Google Scholar]
- 61.Brazitikos PD, Pournaras CJ, Munoz JL, Tsacopoulos M. Microinjection of L-lactate in the preretinal vitreous induces segmental vasodilation in the inner retina of miniature pigs. Invest Ophthalmol Vis Sci 34: 1744–1752, 1993. [PubMed] [Google Scholar]
- 62.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 53: 2646–2655, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bronson-Castain KW, Bearse MA Jr., Neuville J, Jonasdottir S, King-Hooper B, Barez S, Schneck ME, Adams AJ. Adolescents with Type 2 diabetes: early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina (Philadelphia, Pa) 29: 618–626, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brower JB, Targovnik JH, Caplan MR, Massia SP. High glucose-mediated loss of cell surface heparan sulfate proteoglycan impairs the endothelial shear stress response. Cytoskeleton (Hoboken) 67: 135–141, 2010. [DOI] [PubMed] [Google Scholar]
- 65.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med 46: 223–234, 1995. [DOI] [PubMed] [Google Scholar]
- 66.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. In: Diabetes, United States: 2005, p. 1615–1625. [DOI] [PubMed] [Google Scholar]
- 68.Buerk DG, Riva CE and Cranstoun SD. Nitric oxide has a vasodilatory role in cat optic nerve head during flicker stimuli. Microvasc Res 52: 13–26, 1996. [DOI] [PubMed] [Google Scholar]
- 69.Burg MB, Ferraris JD and Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Bursell SE, Clermont AC, Oren B, King GL. The in vivo effect of endothelins on retinal circulation in nondiabetic and diabetic rats. Invest Ophthalmol Vis Sci 36: 596–607, 1995. [PubMed] [Google Scholar]
- 71.Bursell SE, Clermont AC, Shiba T, King GL. Evaluating retinal circulation using video fluorescein angiography in control and diabetic rats. Current eye research 11: 287–295, 1992. [DOI] [PubMed] [Google Scholar]
- 72.Cabrales P, Vazquez BY, Tsai AG, Intaglietta M. Microvascular and capillary perfusion following glycocalyx degradation. J Appl Physiol (1985) 102: 2251–2259, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Cai J and Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (London, England) 16: 242–260, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Cao Y, Feng B, Chen S, Chu Y, Chakrabarti S. Mechanisms of endothelial to mesenchymal transition in the retina in diabetes. Invest Ophthalmol Vis Sci 55: 7321–7331, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Capozzi ME, McCollum GW, Cousins DB, Penn JS. Linoleic Acid is a Diabetes-relevant Stimulator of Retinal Inflammation in Human Retinal Muller Cells and Microvascular Endothelial Cells. J Diabetes Metab 7: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carmo A, Cunha-Vaz JG, Carvalho AP, Lopes MC. L-arginine transport in retinas from streptozotocin diabetic rats: correlation with the level of IL-1 beta and NO synthase activity. Vision research 39: 3817–3823, 1999. [DOI] [PubMed] [Google Scholar]
- 77.Carmo A, Cunha-Vaz JG, Carvalho AP, Lopes MC. Nitric oxide synthase activity in retinas from non-insulin-dependent diabetic Goto-Kakizaki rats: correlation with blood-retinal barrier permeability. Nitric oxide : biology and chemistry 4: 590–596, 2000. [DOI] [PubMed] [Google Scholar]
- 78.Chakrabarti S, Gan XT, Merry A, Karmazyn M, Sima AA. Augmented retinal endothelin-1, endothelin-3, endothelinA and endothelinB gene expression in chronic diabetes. Current eye research 17: 301–307, 1998. [DOI] [PubMed] [Google Scholar]
- 79.Chakravarthy U, Hayes RG, Stitt AW, Douglas A. Endothelin expression in ocular tissues of diabetic and insulin-treated rats. Invest Ophthalmol Vis Sci 38: 2144–2151, 1997. [PubMed] [Google Scholar]
- 80.Chakravarthy U, Hayes RG, Stitt AW, McAuley E, Archer DB. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes 47: 945–952, 1998. [DOI] [PubMed] [Google Scholar]
- 81.Chakravarthy U, Stitt AW, McNally J, Bailie JR, Hoey EM, Duprex P. Nitric oxide synthase activity and expression in retinal capillary endothelial cells and pericytes. Current eye research 14: 285–294, 1995. [DOI] [PubMed] [Google Scholar]
- 82.Chast F and Slama G. [Apollinaire Bouchardat and diabetes]. Hist Sci Med 41: 287–301, 2007. [PubMed] [Google Scholar]
- 83.Chawla A, Chawla R and Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab 20: 546–551, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chaya T, Matsumoto A, Sugita Y, Watanabe S, Kuwahara R, Tachibana M, Furukawa T. Versatile functional roles of horizontal cells in the retinal circuit. Scientific Reports 7: 5540, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheema BS, kohli HS, Sharma R, Bhansali A, Khullar M. Endothelial nitric oxide synthase gene polymorphism and type 2 diabetic retinopathy among Asian Indians. Acta Diabetol 49: 481–488, 2012. [DOI] [PubMed] [Google Scholar]
- 86.Chen Hi HI, Chiang IP and Jen CJ. Exercise Training Increases Acetylcholine-Stimulated Endothelium-Derived Nitric Oxide Release in Spontaneously Hypertensive Rats. Journal of biomedical science 3: 454–460, 1996. [DOI] [PubMed] [Google Scholar]
- 87.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm 2013: 928315, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen YL, Ren Y, Xu W, Rosa RH Jr., Kuo L, Hein TW. Constriction of Retinal Venules to Endothelin-1: Obligatory Roles of ETA Receptors, Extracellular Calcium Entry, and Rho Kinase. Invest Ophthalmol Vis Sci 59: 5167–5175, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose Reductase Deficiency Prevents Diabetes-Induced Blood-Retinal Barrier Breakdown, Apoptosis, and Glial Reactivation in the Retina of db/db Mice. Diabetes 54: 3119–3125, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Chistiakov DA, Orekhov AN and Bobryshev YV. Endothelial PECAM-1 and its function in vascular physiology and atherogenic pathology. Exp Mol Pathol 100: 409–415, 2016. [DOI] [PubMed] [Google Scholar]
- 91.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138: 271–281, 2018. [DOI] [PubMed] [Google Scholar]
- 92.Chong V, Smith RL, Sivaprasad S, Nguyen QD, Rodrigues EBc, Farah ME, Mieler WF. CHAPTER 3 - Retinal biochemistry, physiology, and cell biology. In: Retinal Pharmacotherapy, Edinburgh: W.B. Saunders, 2010, p. 15–22. [Google Scholar]
- 93.Cilensek I, Mankoc S, Globocnik Petrovic M, Petrovic D. The 4a/4a genotype of the VNTR polymorphism for endothelial nitric oxide synthase (eNOS) gene predicts risk for proliferative diabetic retinopathy in Slovenian patients (Caucasians) with type 2 diabetes mellitus. Mol Biol Rep 39: 7061–7067, 2012. [DOI] [PubMed] [Google Scholar]
- 94.Ciulla TA, Amador AG and Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care 26: 2653–2664, 2003. [DOI] [PubMed] [Google Scholar]
- 95.Clermont AC, Aiello LP, Mori F, Aiello LM, Bursell SE. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. American journal of ophthalmology 124: 433–446, 1997. [DOI] [PubMed] [Google Scholar]
- 96.Clermont AC, Brittis M, Shiba T, McGovern T, King GL, Bursell SE. Normalization of retinal blood flow in diabetic rats with primary intervention using insulin pumps. Invest Ophthalmol Vis Sci 35: 981–990, 1994. [PubMed] [Google Scholar]
- 97.Clermont AC and Bursell SE. Retinal blood flow in diabetes. Microcirculation 14: 49–61, 2007. [DOI] [PubMed] [Google Scholar]
- 98.Cogan DG, Toussaint D and Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol 66: 366–378, 1961. [DOI] [PubMed] [Google Scholar]
- 99.Cohan BE and Cohan SB. Flow and oxygen saturation of blood in the anterior ciliary vein of the dog eye. The American journal of physiology 205: 60–66, 1963. [DOI] [PubMed] [Google Scholar]
- 100.Cohen MP and Surma ML. [(35)S]sulfate incorporation into glomerular basement membrane glycosaminoglycans is decreased in experimental diabetes. J Lab Clin Med 98: 715–722, 1981. [PubMed] [Google Scholar]
- 101.Collaborators GRF. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1659–1724, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Collins C, Guilluy C, Welch C, O'Brien ET, Hahn K, Superfine R, Burridge K, Tzima E. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr Biol 22: 2087–2094, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Connell P, Walshe T, Ferguson G, Gao W, O'Brien C, Cahill PA. Elevated glucose attenuates agonist- and flow-stimulated endothelial nitric oxide synthase activity in microvascular retinal endothelial cells. Endothelium : journal of endothelial cell research 14: 17–24, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Conway D and Schwartz MA. Lessons from the endothelial junctional mechanosensory complex. F1000 Biol Rep 4: 1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol 23: 1024–1030, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Coppo R and Amore A. Importance of the bradykinin-nitric oxide synthase system in the hypersensitivity reactions of chronic haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 15: 1288–1290, 2000. [DOI] [PubMed] [Google Scholar]
- 107.Corbalán-García S and Gómez-Fernández JC. Protein kinase C regulatory domains: the art of decoding many different signals in membranes. Biochim Biophys Acta 1761: 633–654, 2006. [DOI] [PubMed] [Google Scholar]
- 108.Country MW. Retinal metabolism: A comparative look at energetics in the retina. Brain Res 1672: 50–57, 2017. [DOI] [PubMed] [Google Scholar]
- 109.Cunha-Vaz JG, Shakib M and Ashton N. Studies on the permeability of the blood-retinal barrier. I. On the existence, development, and site of a blood-retinal barrier. Br J Ophthalmol 50: 441–453, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol 292: 497–523, 1990. [DOI] [PubMed] [Google Scholar]
- 111.Cuthbertson RA and Mandel TE. Anatomy of the mouse retina. Endothelial cell-pericyte ratio and capillary distribution. Invest Ophthalmol Vis Sci 27: 1659–1664, 1986. [PubMed] [Google Scholar]
- 112.Dacey DM, Crook JD and Packer OS. Distinct synaptic mechanisms create parallel S-ON and S-OFF color opponent pathways in the primate retina. Vis Neurosci 31: 139–151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daimon M, Sugiyama K, Saitoh T, Yamaguchi H, Hirata A, Ohnuma H, Igarashi M, Eguchi H, Manaka H, Kato T. Increase in serum ceruloplasmin levels is correlated with a decrease of serum nitric oxide levels in type 2 diabetes. Diabetes Care 23: 559–560, 2000. [DOI] [PubMed] [Google Scholar]
- 114.Danialou G, Vicaut E, Sambe A, Aubier M, Boczkowski J. Predominant role of A1 adenosine receptors in mediating adenosine induced vasodilatation of rat diaphragmatic arterioles: involvement of nitric oxide and the ATP-dependent K+ channels. British journal of pharmacology 121: 1355–1363, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Danser AH, de Vries R, Schoemaker RG, Saxena PR. Bradykinin-induced release of nitric oxide by the isolated perfused rat heart: importance of preformed pools of nitric oxide-containing factors. Journal of hypertension 16: 239–244, 1998. [DOI] [PubMed] [Google Scholar]
- 116.Danser AH, Derkx FH, Admiraal PJ, Deinum J, de Jong PT, Schalekamp MA. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci 35: 1008–1018, 1994. [PubMed] [Google Scholar]
- 117.Danser AH, van den Dorpel MA, Deinum J, Derkx FH, Franken AA, Peperkamp E, de Jong PT, Schalekamp MA. Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. The Journal of clinical endocrinology and metabolism 68: 160–167, 1989. [DOI] [PubMed] [Google Scholar]
- 118.Dasgupta B, Dufour E, Mamdouh Z, Muller WA. A novel and critical role for tyrosine 663 in platelet endothelial cell adhesion molecule-1 trafficking and transendothelial migration. J Immunol 182: 5041–5051, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davis MD. Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol 74: 741–751, 1965. [DOI] [PubMed] [Google Scholar]
- 120.Davis MD. Worsening of diabetic retinopathy after improvement of glycemic control. Arch Ophthalmol 116: 931–932, 1998. [DOI] [PubMed] [Google Scholar]
- 121.Davis MD, Blodi BA, Ryan SJ, Hinton DR, Schachat AP, Wilkinson CP. Chapter 68 - Proliferative Diabetic Retinopathy. In: Retina (Fourth Edition), Edinburgh: Mosby, 2006, p. 1285–1322. [Google Scholar]
- 122.Davisson RL, Bates JN, Johnson AK, Lewis SJ. Use-dependent loss of acetylcholine- and bradykinin-mediated vasodilation after nitric oxide synthase inhibition. Evidence for preformed stores of nitric oxide-containing factors in vascular endothelial cells. Hypertension 28: 354–360, 1996. [DOI] [PubMed] [Google Scholar]
- 123.Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America 88: 7797–7801, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci 47: 5561–5568, 2006. [DOI] [PubMed] [Google Scholar]
- 125.De La Cruz JP, Moreno A, Munoz M, Garcia Campos JM, Sanchez de lC. Effect of aspirin plus dipyridamole on the retinal vascular pattern in experimental diabetes mellitus. J Pharmacol Exp Ther 280: 454–459, 1997. [PubMed] [Google Scholar]
- 126.De La Cruz JP, Moreno A, Ruiz-Ruiz M, Sanchez De La Cuesta F. Effect of DT-TX 30, a combined thromboxane synthase inhibitor and thromboxane receptor antagonist, on retinal vascularity in experimental diabetes mellitus. Thromb Res 97: 125–131, 2000. [DOI] [PubMed] [Google Scholar]
- 127.De La Cruz JP, Moreno A, Ruiz-Ruiz MI, Garcia Campos J, Sanchez de la Cuesta F. Effect of camonagrel, a selective thromboxane synthase inhibitor, on retinal vascularization in experimental diabetes. European journal of pharmacology 350: 81–85, 1998. [DOI] [PubMed] [Google Scholar]
- 128.de Roetth A. Metabolism of the Alloxan Diabetic Rat Retina. Trans Am Ophthalmol Soc 61: 429–458, 1963. [PMC free article] [PubMed] [Google Scholar]
- 129.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5: 261–270, 2004. [DOI] [PubMed] [Google Scholar]
- 130.Dejana E, Bazzoni G and Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res 252: 13–19, 1999. [DOI] [PubMed] [Google Scholar]
- 131.Dejana E and Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci 126: 2545–2549, 2013. [DOI] [PubMed] [Google Scholar]
- 132.Dejana E, Orsenigo F and Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121: 2115–2122, 2008. [DOI] [PubMed] [Google Scholar]
- 133.Delaey C and Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic research 32: 249–256, 2000. [DOI] [PubMed] [Google Scholar]
- 134.Delles C, Michelson G, Harazny J, Oehmer S, Hilgers KF, Schmieder RE. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke 35: 1289–1293, 2004. [DOI] [PubMed] [Google Scholar]
- 135.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (London, England) 20: 1366–1369, 2006. [DOI] [PubMed] [Google Scholar]
- 136.Dene BA, Maritim AC, Sanders RA, Watkins JB 3rd. Effects of antioxidant treatment on normal and diabetic rat retinal enzyme activities. J Ocul Pharmacol Ther 21: 28–35, 2005. [DOI] [PubMed] [Google Scholar]
- 137.Deng D, Evans T, Mukherjee K, Downey D, Chakrabarti S. Diabetes-induced vascular dysfunction in the retina: role of endothelins. Diabetologia 42: 1228–1234, 1999. [DOI] [PubMed] [Google Scholar]
- 138.Deng D and Yan N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci 25: 546–558, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Desjardins C and Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. The American journal of physiology 258: H647–654, 1990. [DOI] [PubMed] [Google Scholar]
- 140.Deussen A, Sonntag M and Vogel R. L-arginine-derived nitric oxide: a major determinant of uveal blood flow. Experimental eye research 57: 129–134, 1993. [DOI] [PubMed] [Google Scholar]
- 141.Devaskar S, Zahm DS, Holtzclaw L, Chundu K, Wadzinski BE. Developmental regulation of the distribution of rat brain insulin-insensitive (Glut 1) glucose transporter. Endocrinology 129: 1530–1540, 1991. [DOI] [PubMed] [Google Scholar]
- 142.Devraj K, Klinger ME, Myers RL, Mokashi A, Hawkins RA, Simpson IA. GLUT-1 glucose transporters in the blood-brain barrier: differential phosphorylation. J Neurosci Res 89: 1913–1925, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Diebel LN, Diebel ME, Martin JV, Liberati DM. Acute hyperglycemia exacerbates trauma-induced endothelial and glycocalyx injury: An in vitro model. J Trauma Acute Care Surg 85: 960–967, 2018. [DOI] [PubMed] [Google Scholar]
- 144.Diebel LN, Liberati DM and Martin JV. Acute hyperglycemia increases sepsis related glycocalyx degradation and endothelial cellular injury: A microfluidic study. Am J Surg 2019. [DOI] [PubMed] [Google Scholar]
- 145.Ding J and Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 12: 346–354, 2012. [DOI] [PubMed] [Google Scholar]
- 146.Dogne S, Rath G, Jouret F, Caron N, Dessy C, Flamion B. Hyaluronidase 1 Deficiency Preserves Endothelial Function and Glycocalyx Integrity in Early Streptozotocin-Induced Diabetes. Diabetes 65: 2742–2753, 2016. [DOI] [PubMed] [Google Scholar]
- 147.Donati G, Pournaras CJ, Munoz JL, Poitry S, Poitry-Yamate CL, Tsacopoulos M. Nitric oxide controls arteriolar tone in the retina of the miniature pig. Invest Ophthalmol Vis Sci 36: 2228–2237, 1995. [PubMed] [Google Scholar]
- 148.Dorner GT, Garhofer G, Huemer KH, Riva CE, Wolzt M, Schmetterer L. Hyperglycemia affects flicker-induced vasodilation in the retina of healthy subjects. Vision research 43: 1495–1500, 2003. [DOI] [PubMed] [Google Scholar]
- 149.Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE, Schmetterer L. Nitric oxide regulates retinal vascular tone in humans. American journal of physiology Heart and circulatory physiology 285: H631–636, 2003. [DOI] [PubMed] [Google Scholar]
- 150.Du Y, Miller CM and Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med 35: 1491–1499, 2003. [DOI] [PubMed] [Google Scholar]
- 151.Du Y, Sarthy VP and Kern TS. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. American journal of physiology Regulatory, integrative and comparative physiology 287: R735–741, 2004. [DOI] [PubMed] [Google Scholar]
- 152.Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. Journal of neurochemistry 80: 771–779, 2002. [DOI] [PubMed] [Google Scholar]
- 153.Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proceedings of the National Academy of Sciences of the United States of America 110: 16586–16591, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dumskyj MJ and Kohner EM. Retinal blood flow regulation in diabetes mellitus: impaired autoregulation and No detectable effect of autonomic neuropathy using laser doppler velocimetry, computer assisted image analysis, and isometric exercise. Microvasc Res 57: 353–356, 1999. [DOI] [PubMed] [Google Scholar]
- 155.Dwyer KJ, Boado RJ and Pardridge WM. Cis-element/cytoplasmic protein interaction within the 3'-untranslated region of the GLUT1 glucose transporter mRNA. Journal of neurochemistry 66: 449–458, 1996. [DOI] [PubMed] [Google Scholar]
- 156.Ebeling P, Koistinen HA and Koivisto VA. Insulin-independent glucose transport regulates insulin sensitivity. FEBS Lett 436: 301–303, 1998. [DOI] [PubMed] [Google Scholar]
- 157.Eelen G, de Zeeuw P, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res 116: 1231–1244, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ehrenfeld P, Millan C, Matus CE, Figueroa JE, Burgos RA, Nualart F, Bhoola KD, Figueroa CD. Activation of kinin B1 receptors induces chemotaxis of human neutrophils. J Leukoc Biol 80: 117–124, 2006. [DOI] [PubMed] [Google Scholar]
- 159.Elgin SS. ARTERIOVENOUS OXYGEN DIFFERENCE ACROSS THE UVEAL TRACT OF THE DOG EYE. Investigative ophthalmology 3: 417–426, 1964. [PubMed] [Google Scholar]
- 160.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. The Journal of biological chemistry 278: 40764–40770, 2003. [DOI] [PubMed] [Google Scholar]
- 161.Eperon G, Johnson M and David NJ. The effect of arterial PO2 on relative retinal blood flow in monkeys. Investigative ophthalmology 14: 342–352, 1975. [PubMed] [Google Scholar]
- 162.Ernest JT, Goldstick TK and Engerman RL. Hyperglycemia impairs retinal oxygen autoregulation in normal and diabetic dogs. Invest Ophthalmol Vis Sci 24: 985–989, 1983. [PubMed] [Google Scholar]
- 163.Eshaq RS and Harris NR. Loss of Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) in the Diabetic Retina: Role of Matrix Metalloproteinases. Invest Ophthalmol Vis Sci 60: 748–760, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eskens BJ, Zuurbier CJ, van Haare J, Vink H, van Teeffelen JW. Effects of two weeks of metformin treatment on whole-body glycocalyx barrier properties in db/db mice. Cardiovasc Diabetol 12: 175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Espaillat A. Diabetic Eye Disease: A Comprehensive Review. Thorofare, NJ: Slack incorporated, 2012. [Google Scholar]
- 166.Esser P, Heimann K and Wiedemann P. Macrophages in proliferative vitreoretinopathy and proliferative diabetic retinopathy: differentiation of subpopulations. Br J Ophthalmol 77: 731–733, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci 15: 507–519, 2014. [DOI] [PubMed] [Google Scholar]
- 168.Faries PL, Rohan DI, Takahara H, Wyers MC, Contreras MA, Quist WC, King GL, Logerfo FW. Human vascular smooth muscle cells of diabetic origin exhibit increased proliferation, adhesion, and migration. J Vasc Surg 33: 601–607, 2001. [DOI] [PubMed] [Google Scholar]
- 169.Feenstra DJ, Yego EC and Mohr S. Modes of Retinal Cell Death in Diabetic Retinopathy. J Clin Exp Ophthalmol 4: 298, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feke GT, Buzney SM, Ogasawara H, Fujio N, Goger DG, Spack NP, Gabbay KH. Retinal circulatory abnormalities in type 1 diabetes. Invest Ophthalmol Vis Sci 35: 2968–2975, 1994. [PubMed] [Google Scholar]
- 171.Feke GT, Zuckerman R, Green GJ, Weiter JJ. Response of human retinal blood flow to light and dark. Invest Ophthalmol Vis Sci 24: 136–141, 1983. [PubMed] [Google Scholar]
- 172.Felder AE, Wanek J, Blair NP, Joslin CE, Brewer KC, Chau FY, Lim JI, Leiderman YI, Shahidi M. The Effects of Diabetic Retinopathy Stage and Light Flicker on Inner Retinal Oxygen Extraction Fraction. Invest Ophthalmol Vis Sci 57: 5586–5592, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fernandes R, Suzuki K and Kumagai AK. Inner blood-retinal barrier GLUT1 in long-term diabetic rats: an immunogold electron microscopic study. Invest Ophthalmol Vis Sci 44: 3150–3154, 2003. [DOI] [PubMed] [Google Scholar]
- 174.Ferrari-Dileo G, Davis EB and Anderson DR. Angiotensin binding sites in bovine and human retinal blood vessels. Invest Ophthalmol Vis Sci 28: 1747–1751, 1987. [PubMed] [Google Scholar]
- 175.Ferrari-Dileo G, Davis EB and Anderson DR. Biochemical evidence for cholinergic activity in retinal blood vessels. Invest Ophthalmol Vis Sci 30: 473–477, 1989. [PubMed] [Google Scholar]
- 176.Ferrari-Dileo G, Ryan JW, Rockwood EJ, Davis EB, Anderson DR. Angiotensin-converting enzyme in bovine, feline, and human ocular tissues. Invest Ophthalmol Vis Sci 29: 876–881, 1988. [PubMed] [Google Scholar]
- 177.Ferrero E, Ferrero ME, Pardi R, Zocchi MR. The platelet endothelial cell adhesion molecule-1 (PECAM1) contributes to endothelial barrier function. FEBS Lett 374: 323–326, 1995. [DOI] [PubMed] [Google Scholar]
- 178.Ferris FL 3rd, Davis MD and Aiello LM. Treatment of diabetic retinopathy. N Engl J Med 341: 667–678, 1999. [DOI] [PubMed] [Google Scholar]
- 179.Ffytche TJ, Bulpitt CJ, Kohner EM, Archer D, Dollery CT. Effect of changes in intraocular pressure on the retinal microcirculation. Br J Ophthalmol 58: 514–522, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fischer F. The First Case of Diabetic Retinopathy. Diabetes Its Medical and Cultural History 363–369, 1989. [Google Scholar]
- 181.Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S. The eye and the heart. European heart journal 34: 1270–1278, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. In: J Cell Sci, England: 2005, vol. Pt 18, p. 4103–4111. [DOI] [PubMed] [Google Scholar]
- 183.Fleming NBB. Case of Retinitis Proliferans without History. Proc R Soc Med 21: 1805–1806, 1928. [PMC free article] [PubMed] [Google Scholar]
- 184.Flynn KM, Michaud M, Canosa S, Madri JA. CD44 regulates vascular endothelial barrier integrity via a PECAM-1 dependent mechanism. Angiogenesis 16: 689–705, 2013. [DOI] [PubMed] [Google Scholar]
- 185.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, Klein R. Retinopathy in diabetes. Diabetes care 27 Suppl 1: S84–87, 2004. [DOI] [PubMed] [Google Scholar]
- 186.Förstermann U and Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 837a-837d, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. 2008. [Google Scholar]
- 188.Frank RN and Ryan S. Chapter 66-Etiologic Mechanisms in Diabetic Retinopathy. In: Retina (4th edition), Philadelphia: Elsevier Mosby, 2006, p. 1241–1270. [Google Scholar]
- 189.Fraser CE and D'Amico DJ. Diabetic Retinopathy: Prevention and treatment. In: UpToDate, edited by Post TW. Waltham, MA: UpToDate, 2019. [Google Scholar]
- 190.Frederiksen CA, Jeppesen P, Knudsen ST, Poulsen PL, Mogensen CE, Bek T. The blood pressure-induced diameter response of retinal arterioles decreases with increasing diabetic maculopathy. Graefes Arch Clin Exp Ophthalmol 244: 1255–1261, 2006. [DOI] [PubMed] [Google Scholar]
- 191.Frye EB, Degenhardt TP, Thorpe SR, Baynes JW. Role of the Maillard reaction in aging of tissue proteins. Advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. The Journal of biological chemistry 273: 18714–18719, 1998. [DOI] [PubMed] [Google Scholar]
- 192.Fuchsjager-Mayrl G, Malec M, Amoako-Mensah T, Kolodjaschna J, Schmetterer L. Changes in choroidal blood flow during light/dark transitions are not altered by atropine or propranolol in healthy subjects. Vision research 43: 2185–2190, 2003. [DOI] [PubMed] [Google Scholar]
- 193.Fuchsjager-Mayrl G, Polska E, Malec M, Schmetterer L. Unilateral light-dark transitions affect choroidal blood flow in both eyes. Vision research 41: 2919–2924, 2001. [DOI] [PubMed] [Google Scholar]
- 194.Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med 288: 831–836, 1973. [DOI] [PubMed] [Google Scholar]
- 195.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008. [DOI] [PubMed] [Google Scholar]
- 196.Galkina E and Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol 17: 368–377, 2006. [DOI] [PubMed] [Google Scholar]
- 197.Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood 102: 169–179, 2003. [DOI] [PubMed] [Google Scholar]
- 198.Garcia-Ramirez M, Hernandez C and Simo R. Expression of erythropoietin and its receptor in the human retina: a comparative study of diabetic and nondiabetic subjects. Diabetes Care 31: 1189–1194, 2008. [DOI] [PubMed] [Google Scholar]
- 199.Garhofer G, Zawinka C, Huemer KH, Schmetterer L, Dorner GT. Flicker light-induced vasodilatation in the human retina: effect of lactate and changes in mean arterial pressure. Invest Ophthalmol Vis Sci 44: 5309–5314, 2003. [DOI] [PubMed] [Google Scholar]
- 200.Garhofer G, Zawinka C, Resch H, Huemer KH, Dorner GT, Schmetterer L. Diffuse luminance flicker increases blood flow in major retinal arteries and veins. Vision research 44: 833–838, 2004. [DOI] [PubMed] [Google Scholar]
- 201.Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol 88: 887–891, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Garhofer G, Zawinka C, Resch H, Menke M, Schmetterer L, Dorner GT. Effect of intravenous administration of sodium-lactate on retinal blood flow in healthy subjects. Invest Ophthalmol Vis Sci 44: 3972–3976, 2003. [DOI] [PubMed] [Google Scholar]
- 203.Garsen M, Rops AL, Rabelink TJ, Berden JH, van der Vlag J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 29: 49–55, 2014. [DOI] [PubMed] [Google Scholar]
- 204.Gencer S, Cebeci A and Irmak-Yazicioglu MB. Matrix metalloproteinase gene expressions might be oxidative stress targets in gastric cancer cell lines. Chin J Cancer Res 25: 322–333, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, Monnier VM. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 54: 3103–3111, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 15: 1298–1306, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Geraldes P and King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106: 1319–1331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gericke A, Goloborodko E, Sniatecki JJ, Steege A, Wojnowski L, Pfeiffer N. Contribution of nitric oxide synthase isoforms to cholinergic vasodilation in murine retinal arterioles. Experimental eye research 109: 60–66, 2013. [DOI] [PubMed] [Google Scholar]
- 209.Gericke A, Wolff I, Musayeva A, Zadeh JK, Manicam C, Pfeiffer N, Li H, Xia N. Retinal arteriole reactivity in mice lacking the endothelial nitric oxide synthase (eNOS) gene. Experimental eye research 181: 150–156, 2019. [DOI] [PubMed] [Google Scholar]
- 210.Geyer O, Neudorfer M, Snir T, Goldstein M, Rock T, Silver DM, Bartov E. Pulsatile ocular blood flow in diabetic retinopathy. Acta ophthalmologica Scandinavica 77: 522–525, 1999. [DOI] [PubMed] [Google Scholar]
- 211.Giacco F and Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Giacco F and Brownlee M. Pathogenesis of Microvascular Complications. Textbook of Diabetes 2010. [Google Scholar]
- 213.Gidday JM, Maceren RG, Shah AR, Meier JA, Zhu Y. KATP channels mediate adenosine-induced hyperemia in retina. Invest Ophthalmol Vis Sci 37: 2624–2633, 1996. [PubMed] [Google Scholar]
- 214.Gidday JM and Park TS. Adenosine-mediated autoregulation of retinal arteriolar tone in the piglet. Invest Ophthalmol Vis Sci 34: 2713–2719, 1993. [PubMed] [Google Scholar]
- 215.Giove TJ, Deshpande MM, Gagen CS, Eldred WD. Increased neuronal nitric oxide synthase activity in retinal neurons in early diabetic retinopathy. Molecular vision 15: 2249–2258, 2009. [PMC free article] [PubMed] [Google Scholar]
- 216.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155: 1624–1638, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Goureau O, Hicks D, Courtois Y, De Kozak Y. Induction and regulation of nitric oxide synthase in retinal Muller glial cells. Journal of neurochemistry 63: 310–317, 1994. [DOI] [PubMed] [Google Scholar]
- 218.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest 109: 383–392, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Granstam E and Granstam SO. Regulation of uveal and retinal blood flow in STZ-diabetic and non-diabetic rats; involvement of nitric oxide. Current eye research 19: 330–337, 1999. [DOI] [PubMed] [Google Scholar]
- 220.Granstam E, Granstam SO, Fellstrom B, Lind L. Endothelium-dependent vasodilation in the uvea of hypertensive and normotensive rats. Current eye research 17: 189–196, 1998. [DOI] [PubMed] [Google Scholar]
- 221.Granstam E, Wang L and Bill A. Ocular effects of endothelin-1 in the cat. Current eye research 11: 325–332, 1992. [DOI] [PubMed] [Google Scholar]
- 222.Grunwald JE, Brucker AJ, Schwartz SS, Braunstein SN, Baker L, Petrig BL, Riva CE. Diabetic glycemic control and retinal blood flow. Diabetes 39: 602–607, 1990. [DOI] [PubMed] [Google Scholar]
- 223.Guthrie D. The History of Diabetes Mellitus, 2nd ed., by N. S. Papaspyros, Stuttgart, G. Thieme Verlag, 1964, pp. 104, 10 plates, revised and supplemented, DM. 19.50. Medical History 9: 198–198, 1965. [Google Scholar]
- 224.Guthrie MJ, Osswald CR and Kang-Mieler JJ. Inverse relationship between the intraretinal concentration of bioavailable nitric oxide and blood glucose in early experimental diabetic retinopathy. Invest Ophthalmol Vis Sci 56: 37–44, 2014. [DOI] [PubMed] [Google Scholar]
- 225.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The Human Microcirculation: Regulation of Flow and Beyond. Circ Res 118: 157–172, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Guyer DR, Schachat AP and Green WR. The choroid: structural considerations. In: Retina (Philadelphia, Pa), edited by Hinton DR. Philadelphia: Elsevier MOSBY, 2006, p. 33–42. [Google Scholar]
- 227.Ha H, Yu MR and Lee HB. High glucose-induced PKC activation mediates TGF-beta 1 and fibronectin synthesis by peritoneal mesothelial cells. Kidney Int 59: 463–470, 2001. [DOI] [PubMed] [Google Scholar]
- 228.Haefliger IO, Flammer J and Lüscher TF. Nitric oxide and endothelin-1 are important regulators of human ophthalmic artery. Invest Ophthalmol Vis Sci 33: 2340–2343, 1992. [PubMed] [Google Scholar]
- 229.Haefliger IO, Meyer P, Flammer J, Lüscher TF. The vascular endothelium as a regulator of the ocular circulation: A new concept in ophthalmology? Survey of ophthalmology 39: 123–132, 1994. [DOI] [PubMed] [Google Scholar]
- 230.Hammer M, Vilser W, Riemer T, Mandecka A, Schweitzer D, Kuhn U, Dawczynski J, Liemt F, Strobel J. Diabetic patients with retinopathy show increased retinal venous oxygen saturation. Graefes Arch Clin Exp Ophthalmol 247: 1025–1030, 2009. [DOI] [PubMed] [Google Scholar]
- 231.Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res 37 Suppl 1: 39–43, 2005. [DOI] [PubMed] [Google Scholar]
- 232.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51: 3107–3112, 2002. [DOI] [PubMed] [Google Scholar]
- 233.Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53: 1104–1110, 2004. [DOI] [PubMed] [Google Scholar]
- 234.Hardy P, Abran D, Hou X, Lahaie I, Peri KG, Asselin P, Varma DR, Chemtob S. A major role for prostacyclin in nitric oxide-induced ocular vasorelaxation in the piglet. Circ Res 83: 721–729, 1998. [DOI] [PubMed] [Google Scholar]
- 235.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci 47: 5106–5115, 2006. [DOI] [PubMed] [Google Scholar]
- 236.Harris A, Bingaman D, Ciulla TA, Martin BJ. Retinal and choroidal blood flow in health and disease. In: Retina (Philadelphia, Pa), edited by Hinton DR. Phildelphia: Elsevier MOSBY, 2006, p. 83–102. [Google Scholar]
- 237.Harris NR. Arteriovenous pairing: a determinant of capillary exchange. News Physiol Sci 18: 83–87, 2003. [DOI] [PubMed] [Google Scholar]
- 238.Harris NR, Leskova W, Kaur G, Eshaq RS, Carter PR. Blood flow distribution and the endothelial surface layer in the diabetic retina. Biorheology 56: 181–189, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Harris NR, Watts MN and Leskova W. Intravital video microscopy measurements of retinal blood flow in mice. J Vis Exp 51110, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hawes NL, Smith RS, Chang B, Davisson M, Heckenlively JR, John SW. Mouse fundus photography and angiography: a catalogue of normal and mutant phenotypes. Molecular vision 5: 22, 1999. [PubMed] [Google Scholar]
- 241.Hein TW, Belardinelli L and Kuo L. Adenosine A2A Receptors Mediate Coronary Microvascular Dilation to Adenosine: Role of Nitric Oxide and ATP-Sensitive Potassium Channels. Journal of Pharmacology and Experimental Therapeutics 291: 655–664, 1999. [PubMed] [Google Scholar]
- 242.Hein TW and Kuo L. cAMP-independent dilation of coronary arterioles to adenosine : role of nitric oxide, G proteins, and K(ATP) channels. Circ Res 85: 634–642, 1999. [DOI] [PubMed] [Google Scholar]
- 243.Hein TW, Potts LB, Xu W, Yuen JZ, Kuo L. Temporal development of retinal arteriolar endothelial dysfunction in porcine type 1 diabetes. Invest Ophthalmol Vis Sci 53: 7943–7949, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hein TW, Ren Y, Potts LB, Yuan Z, Kuo E, Rosa RH Jr., Kuo L. Acute retinal ischemia inhibits endothelium-dependent nitric oxide-mediated dilation of retinal arterioles via enhanced superoxide production. Invest Ophthalmol Vis Sci 53: 30–36, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hein TW, Ren Y, Yuan Z, Xu W, Somvanshi S, Nagaoka T, Yoshida A, Kuo L. Functional and molecular characterization of the endothelin system in retinal arterioles. Invest Ophthalmol Vis Sci 50: 3329–3336, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hein TW, Rosa RH Jr., Yuan Z, Roberts E, Kuo L. Divergent roles of nitric oxide and rho kinase in vasomotor regulation of human retinal arterioles. Invest Ophthalmol Vis Sci 51: 1583–1590, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hein TW, Xu W and Kuo L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci 47: 693–699, 2006. [DOI] [PubMed] [Google Scholar]
- 248.Hein TW, Xu W, Xu X, Kuo L. Acute and Chronic Hyperglycemia Elicit JIP1/JNK-Mediated Endothelial Vasodilator Dysfunction of Retinal Arterioles. Invest Ophthalmol Vis Sci 57: 4333–4340, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hein TW, Yuan Z, Rosa RH Jr., Kuo L. Requisite roles of A2A receptors, nitric oxide, and KATP channels in retinal arteriolar dilation in response to adenosine. Invest Ophthalmol Vis Sci 46: 2113–2119, 2005. [DOI] [PubMed] [Google Scholar]
- 250.Hermans MP, Ahn SA and Rousseau MF. eNOS [Glu298Asp] polymorphism, erectile function and ocular pressure in type 2 diabetes. Eur J Clin Invest 42: 729–737, 2012. [DOI] [PubMed] [Google Scholar]
- 251.Hester RL and Hammer LW. Venular-arteriolar communication in the regulation of blood flow. American journal of physiology Regulatory, integrative and comparative physiology 282: R1280–1285, 2002. [DOI] [PubMed] [Google Scholar]
- 252.Hickam JB, Frayser R and Ross JC. A study of retinal venous blood oxygen saturation in human subjects by photographic means. Circulation 27: 375–385, 1963. [DOI] [PubMed] [Google Scholar]
- 253.Higashi S, Clermont AC, Dhir V, Bursell SE. Reversibility of retinal flow abnormalities is disease-duration dependent in diabetic rats. Diabetes 47: 653–659, 1998. [DOI] [PubMed] [Google Scholar]
- 254.Higgs GA, Moncada S, Salmon JA, Seager K. The source of thromboxane and prostaglandins in experimental inflammation. British journal of pharmacology 79: 863–868, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: E14–22, 2001. [DOI] [PubMed] [Google Scholar]
- 256.Hoeldtke RD, Bryner KD, McNeill DR, Warehime SS, Van Dyke K, Hobbs G. Oxidative stress and insulin requirements in patients with recent-onset type 1 diabetes. The Journal of clinical endocrinology and metabolism 88: 1624–1628, 2003. [DOI] [PubMed] [Google Scholar]
- 257.Hogan MJ and Feeney L. THE ULTRASTRUCTURE OF THE RETINAL BLOOD VESSELS. I. THE LARGE VESSELS. Journal of ultrastructure research 39: 10–28, 1963. [DOI] [PubMed] [Google Scholar]
- 258.Hookes L. Association for Research in Vision and Ophthalmology (ARVO)--2010 Annual Meeting. For Sight: The Future of Eye and Vision Research--part 1. IDrugs 13: 427–429, 2010. [PubMed] [Google Scholar]
- 259.Horio N, Clermont AC, Abiko A, Abiko T, Shoelson BD, Bursell SE, Feener EP. Angiotensin AT(1) receptor antagonism normalizes retinal blood flow and acetylcholine-induced vasodilatation in normotensive diabetic rats. Diabetologia 47: 113–123, 2004. [DOI] [PubMed] [Google Scholar]
- 260.Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 49: 1215–1228, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hoste AM, Boels PJ, Andries LJ, Brutsaert DL, De Laey JJ. Effects of beta-antagonists on contraction of bovine retinal microarteries in vitro. Invest Ophthalmol Vis Sci 31: 1231–1237, 1990. [PubMed] [Google Scholar]
- 262.Hoste AM, Boels PJ, Brutsaert DL, De Laey JJ. Effect of alpha-1 and beta agonists on contraction of bovine retinal resistance arteries in vitro. Invest Ophthalmol Vis Sci 30: 44–50, 1989. [PubMed] [Google Scholar]
- 263.Housey GM, O'Brian CA, Johnson MD, Kirschmeier P, Weinstein IB. Isolation of cDNA clones encoding protein kinase C: evidence for a protein kinase C-related gene family. Proceedings of the National Academy of Sciences of the United States of America 84: 1065–1069, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hu YB, Zhang JK, Sun ZY, Yuan ZG, Liu YM, Mao CJ, Yan H. [Retinal cell apoptosis, microvascular changes and expression of connective tissue growth factor in experimental diabetic rats]. Zhonghua Yan Ke Za Zhi 47: 521–526, 2011. [PubMed] [Google Scholar]
- 265.Huang Q and Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. The American journal of physiology 273: H2442–2451, 1997. [DOI] [PubMed] [Google Scholar]
- 266.Huber AH and Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 105: 391–402, 2001. [DOI] [PubMed] [Google Scholar]
- 267.Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, Merrino VF, Kita S, Iwamoto T, Komuro I, Wang B, Cheung G, Ishikawa E, Ooboshi H, Bader M, Wada K, Kettenmann H, Noda M. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci 27: 13065–13073, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ilan N, Cheung L, Pinter E, Madri JA. Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. The Journal of biological chemistry 275: 21435–21443, 2000. [DOI] [PubMed] [Google Scholar]
- 269.Ilan N, Mahooti S, Rimm DL, Madri JA. PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated beta-catenin. J Cell Sci 112 Pt 18: 3005–3014, 1999. [DOI] [PubMed] [Google Scholar]
- 270.Ilan N, Mohsenin A, Cheung L, Madri JA. PECAM-1 shedding during apoptosis generates a membrane-anchored truncated molecule with unique signaling characteristics. Faseb j 15: 362–372, 2001. [DOI] [PubMed] [Google Scholar]
- 271.Ilhan F and Kalkanli ST. Atherosclerosis and the role of immune cells. World J Clin Cases 3: 345–352, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Illing EK and Gray CH. Retinal metabolism in diabetes; the metabolism of retinae of normal and alloxandiabetic rabbits. J Endocrinol 7: 242–247, 1951. [DOI] [PubMed] [Google Scholar]
- 273.Inagaki Y, Yamagishi S, Okamoto T, Takeuchi M, Amano S. Pigment epithelium-derived factor prevents advanced glycation end products-induced monocyte chemoattractant protein-1 production in microvascular endothelial cells by suppressing intracellular reactive oxygen species generation. Diabetologia 46: 284–287, 2003. [DOI] [PubMed] [Google Scholar]
- 274.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proceedings of the National Academy of Sciences of the United States of America 89: 11059–11063, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proceedings of the National Academy of Sciences of the United States of America 86: 2863–2867, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Insull W. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 122: S3–S14, 2009. [DOI] [PubMed] [Google Scholar]
- 277.Ishibashi T, Tanaka K and Taniguchi Y. Platelet aggregation and coagulation in the pathogenesis of diabetic retinopathy in rats. Diabetes 30: 601–606, 1981. [DOI] [PubMed] [Google Scholar]
- 278.Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y, Adamis AP. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci 44: 2155–2162, 2003. [DOI] [PubMed] [Google Scholar]
- 279.Izumi N, Nagaoka T, Mori F, Sato E, Takahashi A, Yoshida A. Relation between plasma nitric oxide levels and diabetic retinopathy. Jpn J Ophthalmol 50: 465–468, 2006. [DOI] [PubMed] [Google Scholar]
- 280.Jean-Louis S, Lovasik JV and Kergoat H. Systemic hyperoxia and retinal vasomotor responses. Invest Ophthalmol Vis Sci 46: 1714–1720, 2005. [DOI] [PubMed] [Google Scholar]
- 281.Jeppesen P, Aalkjaer C and Bek T. Adenosine relaxation in small retinal arterioles requires functional Na-K pumps and K(ATP) channels. Current eye research 25: 23–28, 2002. [DOI] [PubMed] [Google Scholar]
- 282.Jespersen SN and Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 32: 264–277, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jiang Q, Ding S, Wu J, Liu X, Wu Z. Norepinephrine stimulates mobilization of endothelial progenitor cells after limb ischemia. PLoS One 9: e101774, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Johnson EI, Dunlop ME and Larkins RG. Increased vasodilatory prostaglandin production in the diabetic rat retinal vasculature. Current eye research 18: 79–82, 1999. [DOI] [PubMed] [Google Scholar]
- 285.Joslin EP. Apollinaire Bouchardat 1806–1886. Diabetes Its Medical and Cultural History 359–362, 1989. [Google Scholar]
- 286.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. The American journal of pathology 158: 147–152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. Faseb j 16: 438–440, 2002. [DOI] [PubMed] [Google Scholar]
- 288.Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. The American journal of pathology 160: 501–509, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kadoglou NP, Sailer N, Fotiadis G, Kapelouzou A, Liapis CD. The impact of type 2 diabetes and atorvastatin treatment on serum levels of MMP-7 and MMP-8. Exp Clin Endocrinol Diabetes 122: 44–49, 2014. [DOI] [PubMed] [Google Scholar]
- 290.Kakei Y, Akashi M, Shigeta T, Hasegawa T, Komori T. Alteration of cell-cell junctions in cultured human lymphatic endothelial cells with inflammatory cytokine stimulation. Lymphat Res Biol 12: 136–143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kalantzis G, Angelou M and Poulakou-Rebelakou E. Diabetic retinopathy: an historical assessment. Hormones (Athens) 5: 72–75, 2006. [DOI] [PubMed] [Google Scholar]
- 292.Kalliokoski KK, Knuuti J and Nuutila P. Blood transit time heterogeneity is associated to oxygen extraction in exercising human skeletal muscle. Microvasc Res 67: 125–132, 2004. [DOI] [PubMed] [Google Scholar]
- 293.Kandarakis SA, Piperi C, Topouzis F, Papavassiliou AG. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog Retin Eye Res 42: 85–102, 2014. [DOI] [PubMed] [Google Scholar]
- 294.Kato H, Kuriyama N, Duarte S, Clavien P-A, Busuttil RW, Coito AJ. MMP-9 deficiency shelters endothelial PECAM-1 expression and enhances regeneration of steatotic livers after ischemia and reperfusion injury. Journal of Hepatology 60: 1032–1039, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kaur C, Sivakumar V and Foulds WS. Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci 47: 1126–1141, 2006. [DOI] [PubMed] [Google Scholar]
- 296.Ke Q and Costa M. Hypoxia-inducible factor-1 (HIF-1). Molecular pharmacology 70: 1469–1480, 2006. [DOI] [PubMed] [Google Scholar]
- 297.Kedzierski RM and Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol 41: 851–876, 2001. [DOI] [PubMed] [Google Scholar]
- 298.Kellogg DL Jr., Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol (1985) 98: 629–632, 2005. [DOI] [PubMed] [Google Scholar]
- 299.Kern TS and Engerman RL. Pharmacological inhibition of diabetic retinopathy: aminoguanidine and aspirin. Diabetes 50: 1636–1642, 2001. [DOI] [PubMed] [Google Scholar]
- 300.Kern TS, Miller CM, Du Y, Zheng L, Mohr S, Ball SL, Kim M, Jamison JA, Bingaman DP. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes 56: 373–379, 2007. [DOI] [PubMed] [Google Scholar]
- 301.Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol 279: C21–30, 2000. [DOI] [PubMed] [Google Scholar]
- 302.Kiel JW. Anatomy - The Ocular Circulation. In: The Ocular Circulation: Morgan & Claypool Life Sciences, 2010. [PubMed] [Google Scholar]
- 303.Kiel JW. Choroidal myogenic autoregulation and intraocular pressure. Experimental eye research 58: 529–543, 1994. [DOI] [PubMed] [Google Scholar]
- 304.Kiel JW. Modulation of choroidal autoregulation in the rabbit. Experimental eye research 69: 413–429, 1999. [DOI] [PubMed] [Google Scholar]
- 305.Kiel JW and Shepherd AP. Autoregulation of choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci 33: 2399–2410, 1992. [PubMed] [Google Scholar]
- 306.Kikuchi-Utsumi K, Gao B, Ohinata H, Hashimoto M, Yamamoto N, Kuroshima A. Enhanced gene expression of endothelial nitric oxide synthase in brown adipose tissue during cold exposure. American journal of physiology Regulatory, integrative and comparative physiology 282: R623–626, 2002. [DOI] [PubMed] [Google Scholar]
- 307.Kim JH, Kim JH, Yu YS, Cho CS, Kim KW. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 29: 621–628, 2009. [DOI] [PubMed] [Google Scholar]
- 308.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension 45: 860–866, 2005. [DOI] [PubMed] [Google Scholar]
- 309.Kishi F, Nakaya Y and Ito S. Histamine H2-receptor-mediated nitric oxide release from porcine endothelial cells. Journal of cardiovascular pharmacology 32: 177–182, 1998. [DOI] [PubMed] [Google Scholar]
- 310.Kitada M, Zhang Z, Mima A, King GL. Molecular mechanisms of diabetic vascular complications. J Diabetes Investig 1: 77–89, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kitamura Y, Okamura T, Kani K, Toda N. Nitric oxide-mediated retinal arteriolar and arterial dilatation induced by substance P. Invest Ophthalmol Vis Sci 34: 2859–2865, 1993. [PubMed] [Google Scholar]
- 312.Kizub IV, Klymenko KI and Soloviev AI. Protein kinase C in enhanced vascular tone in diabetes mellitus. Int J Cardiol 174: 230–242, 2014. [DOI] [PubMed] [Google Scholar]
- 313.Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care 18: 258–268, 1995. [DOI] [PubMed] [Google Scholar]
- 314.Knott RM, Robertson M and Forrester JV. Regulation of glucose transporter (GLUT 3) and aldose reductase mRNA inbovine retinal endothelial cells and retinal pericytes in high glucose and high galactose culture. Diabetologia 36: 808–812, 1993. [DOI] [PubMed] [Google Scholar]
- 315.Knowles RG and Moncada S. Nitric oxide synthases in mammals. The Biochemical journal 298 ( Pt 2): 249–258, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Koch KW, Lambrecht HG, Haberecht M, Redburn D, Schmidt HH. Functional coupling of a Ca2+/calmodulin-dependent nitric oxide synthase and a soluble guanylyl cyclase in vertebrate photoreceptor cells. The EMBO journal 13: 3312–3320, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kojima N, Saito M, Mori A, Sakamoto K, Nakahara T, Ishii K. Role of cyclooxygenase in vasodilation of retinal blood vessels induced by bradykinin in Brown Norway rats. Vascul Pharmacol 51: 119–124, 2009. [DOI] [PubMed] [Google Scholar]
- 318.Kolar P. [Patophysiology of diabetic retinopathy]. Vnitr Lek 59: 173–176, 2013. [PubMed] [Google Scholar]
- 319.Kolarova H, Ambruzova B, Svihalkova Sindlerova L, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm 2014: 694312, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Kondo M, Wang L and Bill A. The role of nitric oxide in hyperaemic response to flicker in the retina and optic nerve in cats. Acta ophthalmologica Scandinavica 75: 232–235, 1997. [DOI] [PubMed] [Google Scholar]
- 321.Konno S, Feke GT, Yoshida A, Fujio N, Goger DG, Buzney SM. Retinal blood flow changes in type I diabetes. A long-term follow-up study. Invest Ophthalmol Vis Sci 37: 1140–1148, 1996. [PubMed] [Google Scholar]
- 322.Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Cyclooxygenase pathways. Acta biochimica Polonica 61: 639–649, 2014. [PubMed] [Google Scholar]
- 323.Koss MC. Role of nitric oxide in maintenance of basal anterior choroidal blood flow in rats. Invest Ophthalmol Vis Sci 39: 559–564, 1998. [PubMed] [Google Scholar]
- 324.Kowluru RA. Retinal metabolic abnormalities in diabetic mouse: comparison with diabetic rat. Current eye research 24: 123–128, 2002. [DOI] [PubMed] [Google Scholar]
- 325.Kowluru RA, Atasi L and Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 47: 1594–1599, 2006. [DOI] [PubMed] [Google Scholar]
- 326.Kowluru RA, Kern TS and Engerman RL. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. IV. Antioxidant defense system. Free Radic Biol Med 22: 587–592, 1997. [DOI] [PubMed] [Google Scholar]
- 327.Koya D and King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 47: 859–866, 1998. [DOI] [PubMed] [Google Scholar]
- 328.Krause M, Rodrigues-Krause J, O'Hagan C, De Vito G, Boreham C, Susta D, Newsholme P, Murphy C. Differential nitric oxide levels in the blood and skeletal muscle of type 2 diabetic subjects may be consequence of adiposity: a preliminary study. Metabolism-Clinical and Experimental 61: 1528–1537, 2012. [DOI] [PubMed] [Google Scholar]
- 329.Kumagai AK, Vinores SA and Pardridge WM. Pathological upregulation of inner blood-retinal barrier Glut1 glucose transporter expression in diabetes mellitus. Brain Res 706: 313–317, 1996. [DOI] [PubMed] [Google Scholar]
- 330.Kumase F, Morizane Y, Mohri S, Takasu I, Ohtsuka A, Ohtsuki H. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med Okayama 64: 277–283, 2010. [DOI] [PubMed] [Google Scholar]
- 331.Kur J, Newman EA and Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res 31: 377–406, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Laios K, Karamanou M, Saridaki Z, Androutsos G. Aretaeus of Cappadocia and the first description of diabetes. Hormones (Athens) 11: 109–113, 2012. [DOI] [PubMed] [Google Scholar]
- 333.Langham ME, Grebe R, Hopkins S, Marcus S, Sebag M. Choroidal blood flow in diabetic retinopathy. Experimental eye research 52: 167–173, 1991. [DOI] [PubMed] [Google Scholar]
- 334.Lantoine F, Iouzalen L, Devynck MA, Millanvoye-Van Brussel E, David-Dufilho M. Nitric oxide production in human endothelial cells stimulated by histamine requires Ca2+ influx. The Biochemical journal 330 ( Pt 2): 695–699, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Laspas P, Goloborodko E, Sniatecki JJ, Kordasz ML, Manicam C, Wojnowski L, Li H, Patzak A, Pfeiffer N, Gericke A. Role of nitric oxide synthase isoforms for ophthalmic artery reactivity in mice. Experimental eye research 127: 1–8, 2014. [DOI] [PubMed] [Google Scholar]
- 336.Lasta M, Pemp B, Schmidl D, Boltz A, Kaya S, Palkovits S, Werkmeister R, Howorka K, Popa-Cherecheanu A, Garhofer G, Schmetterer L. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Invest Ophthalmol Vis Sci 54: 842–847, 2013. [DOI] [PubMed] [Google Scholar]
- 337.Laties AM. Central retinal artery innervation. Absence of adrenergic innervation to the intraocular branches. Arch Ophthalmol 77: 405–409, 1967. [DOI] [PubMed] [Google Scholar]
- 338.Lau JC and Linsenmeier RA. Increased intraretinal PO2 in short-term diabetic rats. Diabetes 63: 4338–4342, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lau JC and Linsenmeier RA. Oxygen Consumption and Distribution in the Diabetic Rat Retina. Invest Ophthalmol Vis Sci 51: E-abstract 5644, 2010. [Google Scholar]
- 340.Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrosio AF, Forrester JV. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci 48: 5257–5265, 2007. [DOI] [PubMed] [Google Scholar]
- 341.Lecleire-Collet A, Audo I, Aout M, Girmens JF, Sofroni R, Erginay A, Le Gargasson JF, Mohand-Said S, Meas T, Guillausseau PJ, Vicaut E, Paques M, Massin P. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci 52: 2861–2867, 2011. [DOI] [PubMed] [Google Scholar]
- 342.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130: 691–703, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lee S and Harris NR. Losartan and ozagrel reverse retinal arteriolar constriction in non-obese diabetic mice. Microcirculation 15: 379–387, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lee S, Morgan GA and Harris NR. Ozagrel reverses streptozotocin-induced constriction of arterioles in rat retina. Microvasc Res 76: 217–223, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lee SB, Bae IH, Bae YS, Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. The Journal of biological chemistry 281: 36228–36235, 2006. [DOI] [PubMed] [Google Scholar]
- 346.Leskova W, Pickett H, Eshaq RS, Shrestha B, Pattillo CB, Harris NR. Effect of diabetes and hyaluronidase on the retinal endothelial glycocalyx in mice. Experimental eye research 179: 125–131, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Leskova W, Watts MN, Carter PR, Eshaq RS, Harris NR. Measurement of retinal blood flow rate in diabetic rats: disparity between techniques due to redistribution of flow. Invest Ophthalmol Vis Sci 54: 2992–2999, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Ley K. The role of selectins in inflammation and disease. Trends Mol Med 9: 263–268, 2003. [DOI] [PubMed] [Google Scholar]
- 349.Li C, Chen P, Zhang J, Zhang L, Huang X, Yao Y, Che X, Fan X, Ge S, Wang Z. Enzyme-induced vitreolysis can alleviate the progression of diabetic retinopathy through the HIF-1alpha pathway. Invest Ophthalmol Vis Sci 54: 4964–4970, 2013. [DOI] [PubMed] [Google Scholar]
- 350.Li H, Bui BV, Cull G, Wang F, Wang L. Glial Cell Contribution to Basal Vessel Diameter and Pressure-Initiated Vascular Responses in Rat Retina. Invest Ophthalmol Vis Sci 58: 1–8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Li H, Burkhardt C, Heinrich UR, Brausch I, Xia N, Forstermann U. Histamine upregulates gene expression of endothelial nitric oxide synthase in human vascular endothelial cells. Circulation 107: 2348–2354, 2003. [DOI] [PubMed] [Google Scholar]
- 352.Li Q and Puro DG. Adenosine activates ATP-sensitive K(+) currents in pericytes of rat retinal microvessels: role of A1 and A2a receptors. Brain Res 907: 93–99, 2001. [DOI] [PubMed] [Google Scholar]
- 353.Li Q, Verma A, Han PY, Nakagawa T, Johnson RJ, Grant MB, Campbell-Thompson M, Jarajapu YP, Lei B, Hauswirth WW. Diabetic eNOS-knockout mice develop accelerated retinopathy. Invest Ophthalmol Vis Sci 51: 5240–5246, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Li W, Yanoff M, Liu X, Ye X. Retinal capillary pericyte apoptosis in early human diabetic retinopathy. Chin Med J (Engl) 110: 659–663, 1997. [PubMed] [Google Scholar]
- 355.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med 182: 1337–1343, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Lim LS, Ling LH, Ong PG, Foulds W, Tai ES, Wong E, Lee SY, Wong D, Cheung CM, Wong TY. Dynamic responses in retinal vessel caliber with flicker light stimulation in eyes with diabetic retinopathy. Invest Ophthalmol Vis Sci 55: 5207–5213, 2014. [DOI] [PubMed] [Google Scholar]
- 357.Limaye V, Li X, Hahn C, Xia P, Berndt MC, Vadas MA, Gamble JR. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. In: Blood, United States: 2005, vol. 8, p. 3169–3177. [DOI] [PubMed] [Google Scholar]
- 358.Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol 88: 521–542, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Linsenmeier RA and Braun RD. Oxygen distribution and consumption in the cat retina during normoxia and hypoxemia. J Gen Physiol 99: 177–197, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, Hatchell DL, McLeod DS, Lutty GA. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci 39: 1647–1657, 1998. [PubMed] [Google Scholar]
- 361.Lipowsky HH. Protease Activity and the Role of the Endothelial Glycocalyx in Inflammation. Drug Discov Today Dis Models 8: 57–62, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Liu J and Feener EP. Plasma kallikrein-kinin system and diabetic retinopathy. Biol Chem 394: 319–328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Ljungkvist AS, Bussink J, Kaanders JH, van der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiation research 167: 127–145, 2007. [DOI] [PubMed] [Google Scholar]
- 364.Lo TW, Westwood ME, McLellan AC, Selwood T, Thornalley PJ. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. The Journal of biological chemistry 269: 32299–32305, 1994. [PubMed] [Google Scholar]
- 365.Lopez-Quintero SV, Cancel LM, Pierides A, Antonetti D, Spray DC, Tarbell JM. High glucose attenuates shear-induced changes in endothelial hydraulic conductivity by degrading the glycocalyx. PLoS One 8: e78954, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res 2007: 61038, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Losiewicz MK and Fort PE. Diabetes impairs the neuroprotective properties of retinal alpha-crystallins. Invest Ophthalmol Vis Sci 52: 5034–5042, 2011. [DOI] [PubMed] [Google Scholar]
- 368.Lowry OH, Roberts NR and Lewis C. The quantitative histochemistry of the retina. The Journal of biological chemistry 220: 879–892, 1956. [PubMed] [Google Scholar]
- 369.Lutjen-Drecoll E. Choroidal innervation in primate eyes. Experimental eye research 82: 357–361, 2006. [DOI] [PubMed] [Google Scholar]
- 370.Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, Fletcher EL. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci 52: 9316–9326, 2011. [DOI] [PubMed] [Google Scholar]
- 371.Ma ZJ, Chen R, Ren HZ, Guo X, Guo J, Chen LM. Association between eNOS 4b/a polymorphism and the risk of diabetic retinopathy in type 2 diabetes mellitus: a meta-analysis. J Diabetes Res 2014: 549747, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.MacKinnon JR, O'Brien C, Swa K, Aspinall P, Butt Z, Cameron D. Pulsatile ocular blood flow in untreated diabetic retinopathy. Acta ophthalmologica Scandinavica 75: 661–664, 1997. [DOI] [PubMed] [Google Scholar]
- 373.Madge LA and Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol 70: 317–325, 2001. [DOI] [PubMed] [Google Scholar]
- 374.Maeda S, Matsui T, Ojima A, Takeuchi M, Yamagishi S. Sulforaphane inhibits advanced glycation end product-induced pericyte damage by reducing expression of receptor for advanced glycation end products. Nutr Res 34: 807–813, 2014. [DOI] [PubMed] [Google Scholar]
- 375.Malecaze F, Clamens S, Simorre-Pinatel V, Mathis A, Chollet P, Favard C, Bayard F, Plouet J. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol 112: 1476–1482, 1994. [DOI] [PubMed] [Google Scholar]
- 376.Mandecka A, Dawczynski J, Blum M, Muller N, Kloos C, Wolf G, Vilser W, Hoyer H, Muller UA. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care 30: 3048–3052, 2007. [DOI] [PubMed] [Google Scholar]
- 377.Manea SA, Robciuc A, Guja C, Heltianu C. Identification of gene variants in NOS3, ET-1 and RAS that confer risk and protection against microangiopathy in type 2 diabetic obese subjects. Biochemical and biophysical research communications 407: 486–490, 2011. [DOI] [PubMed] [Google Scholar]
- 378.Marasciulo FL, Montagnani M and Potenza MA. Endothelin-1: the yin and yang on vascular function. Curr Med Chem 13: 1655–1665, 2006. [DOI] [PubMed] [Google Scholar]
- 379.Mariani AP. The neuronal organization of the outer plexiform layer of the primate retina. Int Rev Cytol 86: 285–320, 1984. [DOI] [PubMed] [Google Scholar]
- 380.Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 45: 3330–3336, 2004. [DOI] [PubMed] [Google Scholar]
- 381.Massey SC. Functional Anatomy of the Mammalian Retina. In: Retina (Philadelphia, Pa), edited by Hinton DR. Philadelphia: Elsevier MOSBY, 2006, vol. 1, p. 43–82. [Google Scholar]
- 382.Maurer H and Gerber G. [The central retinal artery of the white laboratory mouse]. Anatomischer Anzeiger 162: 233–240, 1986. [PubMed] [Google Scholar]
- 383.McClatchey PM, Schafer M, Hunter KS, Reusch JE. The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. American journal of physiology Heart and circulatory physiology 311: H168–176, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.McLeod D. Why cotton wool spots should not be regarded as retinal nerve fibre layer infarcts. British Journal of Ophthalmology 89: 229, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.McLeod D, Marshall J, Kohner EM, Bird AC. The role of axoplasmic transport in the pathogenesis of retinal cotton-wool spots. Br J Ophthalmol 61: 177–191, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. The American journal of pathology 147: 642–653, 1995. [PMC free article] [PubMed] [Google Scholar]
- 387.Metea MR and Newman EA. Signalling within the neurovascular unit in the mammalian retina. Experimental physiology 92: 635–640, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Meyer P, Champion C, Schlotzer-Schrehardt U, Flammer J, Haefliger IO. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Current eye research 18: 375–380, 1999. [DOI] [PubMed] [Google Scholar]
- 389.Michel CC and Curry FR. Glycocalyx volume: a critical review of tracer dilution methods for its measurement. Microcirculation 16: 213–219, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Minagar A and Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler 9: 540–549, 2003. [DOI] [PubMed] [Google Scholar]
- 391.Mishra A and Newman EA. Aminoguanidine reverses the loss of functional hyperemia in a rat model of diabetic retinopathy. Frontiers in neuroenergetics 3: 10, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Mishra A and Newman EA. Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia 58: 1996–2004, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Mizutani M, Kern TS and Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 97: 2883–2890, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Momeni A, Chaleshtori MH, Saadatmand S, Kheiri S. Correlation of Endothelial Nitric Oxide Synthase Gene Polymorphism (GG, TT and GT Genotype) with Proteinuria and Retinopathy in Type 2 Diabetic Patients. J Clin Diagn Res 10: OC32–35, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Moore KJ and Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 145: 341–355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Moore TC, Moore JE, Kaji Y, Frizzell N, Usui T, Poulaki V, Campbell IL, Stitt AW, Gardiner TA, Archer DB, Adamis AP. The role of advanced glycation end products in retinal microvascular leukostasis. Invest Ophthalmol Vis Sci 44: 4457–4464, 2003. [DOI] [PubMed] [Google Scholar]
- 397.Moret P, Pournaras CJ, Munoz JL, Brazitikos P, Tsacopoulos M. [Profile of pO2. I. Profile of transretinal pO2 in hypoxia]. Klinische Monatsblatter fur Augenheilkunde 200: 498–499, 1992. [DOI] [PubMed] [Google Scholar]
- 398.Mori A, Saito M, Sakamoto K, Nakahara T, Ishii K. Intravenously administered vasodilatory prostaglandins increase retinal and choroidal blood flow in rats. J Pharmacol Sci 103: 103–112, 2007. [DOI] [PubMed] [Google Scholar]
- 399.Mori F, King GL, Clermont AC, Bursell DK, Bursell SE. Endothelin-3 regulation of retinal hemodynamics in nondiabetic and diabetic rats. Invest Ophthalmol Vis Sci 41: 3955–3962, 2000. [PubMed] [Google Scholar]
- 400.Moscat J and Diaz-Meco MT. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep 1: 399–403, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Motta G, Rojkjaer R, Hasan AA, Cines DB, Schmaier AH. High molecular weight kininogen regulates prekallikrein assembly and activation on endothelial cells: a novel mechanism for contact activation. Blood 91: 516–528, 1998. [PubMed] [Google Scholar]
- 402.Mukherjee A, Roy S, Saha B, Mukherjee D. Spatio-Temporal Regulation of PKC Isoforms Imparts Signaling Specificity. Front Immunol 7: 45, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol 24: 327–334, 2003. [DOI] [PubMed] [Google Scholar]
- 404.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 6: 323–344, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med 178: 449–460, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest 74: 819–825, 1996. [PubMed] [Google Scholar]
- 407.Mylona-Karayanni C, Gourgiotis D, Bossios A, Kamper EF. Oxidative stress and adhesion molecules in children with type 1 diabetes mellitus: a possible link. Pediatr Diabetes 7: 51–59, 2006. [DOI] [PubMed] [Google Scholar]
- 408.Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, Yamashiro K, Inoue M, Tsubota K, Umezawa K, Ishida S. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci 48: 4342–4350, 2007. [DOI] [PubMed] [Google Scholar]
- 409.Naganuma Y, Satoh K, Yi Q, Asazuma N, Yatomi Y, Ozaki Y. Cleavage of platelet endothelial cell adhesion molecule-1 (PECAM-1) in platelets exposed to high shear stress. J Thromb Haemost 2: 1998–2008, 2004. [DOI] [PubMed] [Google Scholar]
- 410.Nagaoka T, Kuo L, Ren Y, Yoshida A, Hein TW. C-reactive protein inhibits endothelium-dependent nitric oxide-mediated dilation of retinal arterioles via enhanced superoxide production. Invest Ophthalmol Vis Sci 49: 2053–2060, 2008. [DOI] [PubMed] [Google Scholar]
- 411.Nagaoka T, Sakamoto T, Mori F, Sato E, Yoshida A. The effect of nitric oxide on retinal blood flow during hypoxia in cats. Invest Ophthalmol Vis Sci 43: 3037–3044, 2002. [PubMed] [Google Scholar]
- 412.Nakazawa T, Mori A, Saito M, Sakamoto K, Nakahara T, Ishii K. Vasodilator effects of adenosine on retinal arterioles in streptozotocin-induced diabetic rats. Naunyn-Schmiedeberg's archives of pharmacology 376: 423–430, 2008. [DOI] [PubMed] [Google Scholar]
- 413.Narne P, Ponnaluri KC, Siraj M, Ishaq M. Association Analysis of Polymorphisms in Genes Related to Oxidative Stress in South Indian Type 2 Diabetic Patients with Retinopathy. Ophthalmic Genet 37: 1–8, 2016. [DOI] [PubMed] [Google Scholar]
- 414.Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993. [DOI] [PubMed] [Google Scholar]
- 415.Nelissen I, Gveric D, van Noort JM, Cuzner ML, Opdenakker G. PECAM-1 and gelatinase B coexist in vascular cuffs of multiple sclerosis lesions. Neuropathol Appl Neurobiol 32: 15–22, 2006. [DOI] [PubMed] [Google Scholar]
- 416.Nesmith BL, Palacio AC, Schaal Y, Gupta A, Schaal S. DIABETES ALTERS THE MAGNITUDE OF VITREOMACULAR ADHESION. Retina (Philadelphia, Pa) 37: 749–752, 2017. [DOI] [PubMed] [Google Scholar]
- 417.Newman PJ. The role of PECAM-1 in vascular cell biology. Ann N Y Acad Sci 714: 165–174, 1994. [DOI] [PubMed] [Google Scholar]
- 418.Newman PJ and Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol 23: 953–964, 2003. [DOI] [PubMed] [Google Scholar]
- 419.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. The Biochemical journal 370: 361–371, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Nguyen TT, Kawasaki R, Wang JJ, Kreis AJ, Shaw J, Vilser W, Wong TY. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care 32: 2075–2080, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Nguyen TT, Shaw JE, Robinson C, Kawasaki R, Wang JJ, Kreis AJ, Wong TY. Diabetic retinopathy is related to both endothelium-dependent and -independent responses of skin microvascular flow. Diabetes Care 34: 1389–1393, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Nielsen PJ and Nyborg NC. Contractile and relaxing effects of arachidonic acid derivatives on isolated bovine retinal resistance arteries. Experimental eye research 50: 305–311, 1990. [DOI] [PubMed] [Google Scholar]
- 423.Nieuwdorp M, Holleman F, de Groot E, Vink H, Gort J, Kontush A, Chapman MJ, Hutten BA, Brouwer CB, Hoekstra JB, Kastelein JJ, Stroes ES. Perturbation of hyaluronan metabolism predisposes patients with type 1 diabetes mellitus to atherosclerosis. Diabetologia 50: 1288–1293, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55: 1127–1132, 2006. [DOI] [PubMed] [Google Scholar]
- 425.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55: 480–486, 2006. [DOI] [PubMed] [Google Scholar]
- 426.Nilsson M, von Wendt G, Wanger P, Martin L. Early detection of macular changes in patients with diabetes using Rarebit Fovea Test and optical coherence tomography. Br J Ophthalmol 91: 1596–1598, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Nishikawa T, Edelstein D and Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl 77: S26–30, 2000. [DOI] [PubMed] [Google Scholar]
- 428.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000. [DOI] [PubMed] [Google Scholar]
- 429.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 334: 661–665, 1988. [DOI] [PubMed] [Google Scholar]
- 430.Noble KE, Wickremasinghe RG, DeCornet C, Panayiotidis P, Yong KL. Monocytes stimulate expression of the Bcl-2 family member, A1, in endothelial cells and confer protection against apoptosis. J Immunol 162: 1376–1383, 1999. [PubMed] [Google Scholar]
- 431.Noda K, Ishida S, Inoue M, Obata K, Oguchi Y, Okada Y, Ikeda E. Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 44: 2163–2170, 2003. [DOI] [PubMed] [Google Scholar]
- 432.Noyes HD. Retinitis in Glycosuria. Trans Am Ophthalmol Soc 1: 71–75, 1868. [PMC free article] [PubMed] [Google Scholar]
- 433.Nusing R, Lesch R and Ullrich V. Immunohistochemical localization of thromboxane synthase in human tissues. Eicosanoids 3: 53–58, 1990. [PubMed] [Google Scholar]
- 434.Nussbaum C, Cavalcanti Fernandes Heringa A, Mormanova Z, Puchwein-Schwepcke AF, Bechtold-Dalla Pozza S, Genzel-Boroviczeny O. Early microvascular changes with loss of the glycocalyx in children with type 1 diabetes. J Pediatr 164: 584–589 e581, 2014. [DOI] [PubMed] [Google Scholar]
- 435.Nuzum DS and Merz T. Macrovascular Complications of Diabetes Mellitus. Journal of pharmacy practice 22: 135–148, 2009. [Google Scholar]
- 436.Obrosova IG, Drel VR, Kumagai AK, Szabo C, Pacher P, Stevens MJ. Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia 49: 2525–2533, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Obrosova IG, Fathallah L and Greene DA. Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina: effect of DL-alpha-lipoic acid. European journal of pharmacology 398: 139–146, 2000. [DOI] [PubMed] [Google Scholar]
- 438.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. The Journal of biological chemistry 276: 29596–29602, 2001. [DOI] [PubMed] [Google Scholar]
- 439.Oku H, Kida T, Sugiyama T, Hamada J, Sato B, Ikeda T. Possible involvement of endothelin-1 and nitric oxide in the pathogenesis of proliferative diabetic retinopathy. Retina (Philadelphia, Pa) 21: 647–651, 2001. [DOI] [PubMed] [Google Scholar]
- 440.Olney JW. An electron microscopic study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Investigative ophthalmology 7: 250–268, 1968. [PubMed] [Google Scholar]
- 441.Omri S, Omri B, Savoldelli M, Jonet L, Thillaye-Goldenberg B, Thuret G, Gain P, Jeanny JC, Crisanti P, Behar-Cohen F. The outer limiting membrane (OLM) revisited: clinical implications. Clin Ophthalmol 4: 183–195, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol 158: 773–785, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Osborne NN, Barnett NL and Herrera AJ. NADPH diaphorase localization and nitric oxide synthetase activity in the retina and anterior uvea of the rabbit eye. Brain Res 610: 194–198, 1993. [DOI] [PubMed] [Google Scholar]
- 444.Ostergaard L, Kristiansen SB, Angleys H, Frokiaer J, Michael Hasenkam J, Jespersen SN, Botker HE. The role of capillary transit time heterogeneity in myocardial oxygenation and ischemic heart disease. Basic Res Cardiol 109: 409, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Otani S, Nagaoka T, Omae T, Tanano I, Kamiya T, Ono S, Hein TW, Kuo L, Yoshida A. Histamine-Induced Dilation of Isolated Porcine Retinal Arterioles: Role of Endothelium-Derived Hyperpolarizing Factor. Invest Ophthalmol Vis Sci 57: 4791–4798, 2016. [DOI] [PubMed] [Google Scholar]
- 446.Ozawa M, Baribault H and Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. The EMBO journal 8: 1711–1717, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Ozden S, Tatlipinar S, Bicer N, Yaylali V, Yildirim C, Ozbay D, Guner G. Basal serum nitric oxide levels in patients with type 2 diabetes mellitus and different stages of retinopathy. Can J Ophthalmol 38: 393–396, 2003. [DOI] [PubMed] [Google Scholar]
- 448.Page-McCaw A, Ewald AJ and Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8: 221–233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Pahwa R, Nallasamy P and Jialal I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J Diabetes Complications 30: 563–572, 2016. [DOI] [PubMed] [Google Scholar]
- 450.Paques M, Tadayoni R, Sercombe R, Laurent P, Genevois O, Gaudric A, Vicaut E. Structural and hemodynamic analysis of the mouse retinal microcirculation. Invest Ophthalmol Vis Sci 44: 4960–4967, 2003. [DOI] [PubMed] [Google Scholar]
- 451.Park JW, Park SJ, Park SH, Kim KY, Chung JW, Chun MH, Oh SJ. Up-regulated expression of neuronal nitric oxide synthase in experimental diabetic retina. Neurobiology of disease 21: 43–49, 2006. [DOI] [PubMed] [Google Scholar]
- 452.Park JY, Takahara N, Gabriele A, Chou E, Naruse K, Suzuma K, Yamauchi T, Ha SW, Meier M, Rhodes CJ, King GL. Induction of endothelin-1 expression by glucose: an effect of protein kinase C activation. Diabetes 49: 1239–1248, 2000. [DOI] [PubMed] [Google Scholar]
- 453.Park SH, Park JW, Park SJ, Kim KY, Chung JW, Chun MH, Oh SJ. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia 46: 1260–1268, 2003. [DOI] [PubMed] [Google Scholar]
- 454.Parver LM, Auker C and Carpenter DO. Choroidal blood flow as a heat dissipating mechanism in the macula. American journal of ophthalmology 89: 641–646, 1980. [DOI] [PubMed] [Google Scholar]
- 455.Parver LM, Auker CR and Carpenter DO. The stabilizing effect of the choroidal circulation on the temperature environment of the macula. Retina (Philadelphia, Pa) 2: 117–120, 1982. [DOI] [PubMed] [Google Scholar]
- 456.Patel JI, Saleh GM, Hykin PG, Gregor ZJ, Cree IA. Concentration of haemodynamic and inflammatory related cytokines in diabetic retinopathy. Eye (London, England) 22: 223–228, 2008. [DOI] [PubMed] [Google Scholar]
- 457.Patel SA and Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell death and differentiation 15: 628–634, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Patel V, Rassam S, Newsom R, Wiek J, Kohner E. Retinal blood flow in diabetic retinopathy. BMJ (Clinical research ed) 305: 678–683, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Paterno R, Faraci FM and Heistad DD. Role of Ca(2+)-dependent K+ channels in cerebral vasodilatation induced by increases in cyclic GMP and cyclic AMP in the rat. Stroke 27: 1603–1607; discussion 1607-1608, 1996. [DOI] [PubMed] [Google Scholar]
- 460.Pemp B, Garhofer G, Weigert G, Karl K, Resch H, Wolzt M, Schmetterer L. Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Invest Ophthalmol Vis Sci 50: 4029–4032, 2009. [DOI] [PubMed] [Google Scholar]
- 461.Phipps JA and Feener EP. The kallikrein-kinin system in diabetic retinopathy: lessons for the kidney. Kidney Int 73: 1114–1119, 2008. [DOI] [PubMed] [Google Scholar]
- 462.Pinna A, Emanueli C, Dore S, Salvo M, Madeddu P, Carta F. Levels of human tissue kallikrein in the vitreous fluid of patients with severe proliferative diabetic retinopathy. Ophthalmologica 218: 260–263, 2004. [DOI] [PubMed] [Google Scholar]
- 463.Polak K, Luksch A, Frank B, Jandrasits K, Polska E, Schmetterer L. Regulation of human retinal blood flow by endothelin-1. Experimental eye research 76: 633–640, 2003. [DOI] [PubMed] [Google Scholar]
- 464.Polak K, Schmetterer L and Riva CE. Influence of flicker frequency on flicker-induced changes of retinal vessel diameter. Invest Ophthalmol Vis Sci 43: 2721–2726, 2002. [PubMed] [Google Scholar]
- 465.Popov D. Endothelial cell dysfunction in hyperglycemia: Phenotypic change, intracellular signaling modification, ultrastructural alteration, and potential clinical outcomes. International Journal of Diabetes Mellitus 2: 189–195, 2010. [Google Scholar]
- 466.Potts LB, Bradley PD, Xu W, Kuo L, Hein TW. Role of endothelium in vasomotor responses to endothelin system and protein kinase C activation in porcine retinal arterioles. Invest Ophthalmol Vis Sci 54: 7587–7594, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Poulaki V, Joussen AM, Mitsiades N, Mitsiades CS, Iliaki EF, Adamis AP. Insulin-like growth factor-I plays a pathogenetic role in diabetic retinopathy. The American journal of pathology 165: 457–469, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest 109: 805–815, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Pouliot M, Talbot S, Senecal J, Dotigny F, Vaucher E, Couture R. Ocular application of the kinin B1 receptor antagonist LF22-0542 inhibits retinal inflammation and oxidative stress in streptozotocin-diabetic rats. PLoS One 7: e33864, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Pournaras C, Tsacopoulos M and Chapuis P. Studies on the role of prostaglandins in the regulation of retinal blood flow. Experimental eye research 26: 687–697, 1978. [DOI] [PubMed] [Google Scholar]
- 471.Pournaras CJ. Retinal oxygen distribution. Its role in the physiopathology of vasoproliferative microangiopathies. Retina (Philadelphia, Pa) 15: 332–347, 1995. [PubMed] [Google Scholar]
- 472.Pournaras CJ, Riva CE, Tsacopoulos M, Strommer K. Diffusion of O2 in the retina of anesthetized miniature pigs in normoxia and hyperoxia. Experimental eye research 49: 347–360, 1989. [DOI] [PubMed] [Google Scholar]
- 473.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res 27: 284–330, 2008. [DOI] [PubMed] [Google Scholar]
- 474.Powell ED and Field RA. DIABETIC RETINOPATHY AND RHEUMATOID ARTHRITIS. Lancet 2: 17–18, 1964. [DOI] [PubMed] [Google Scholar]
- 475.Pricci F, Leto G, Amadio L, Iacobini C, Cordone S, Catalano S, Zicari A, Sorcini M, Di Mario U, Pugliese G. Oxidative stress in diabetes-induced endothelial dysfunction involvement of nitric oxide and protein kinase C. Free Radic Biol Med 35: 683–694, 2003. [DOI] [PubMed] [Google Scholar]
- 476.Privratsky JR, Paddock CM, Florey O, Newman DK, Muller WA, Newman PJ. Relative contribution of PECAM-1 adhesion and signaling to the maintenance of vascular integrity. In: J Cell Sci, England: 2011, vol. Pt 9, p. 1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Pruneau D, Belichard P, Sahel JA, Combal JP. Targeting the kallikrein-kinin system as a new therapeutic approach to diabetic retinopathy. Curr Opin Investig Drugs 11: 507–514, 2010. [PubMed] [Google Scholar]
- 478.Qiao YC, Chen YL, Pan YH, Tian F, Xu Y, Zhang XX, Zhao HL. The change of serum tumor necrosis factor alpha in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. PLoS One 12: e0176157, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Rajah TT, Olson AL and Grammas P. Differential glucose uptake in retina- and brain-derived endothelial cells. Microvasc Res 62: 236–242, 2001. [DOI] [PubMed] [Google Scholar]
- 480.Rask-Madsen C and King GL. Differential regulation of VEGF signaling by PKC-alpha and PKC-epsilon in endothelial cells. Arterioscler Thromb Vasc Biol 28: 919–924, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Rask-Madsen C and King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 17: 20–33, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Razandi M, Pedram A, Rubin T, Levin ER. PGE2 and PGI2 inhibit ET-1 secretion from endothelial cells by stimulating particulate guanylate cyclase. The American journal of physiology 270: H1342–1349, 1996. [DOI] [PubMed] [Google Scholar]
- 483.Reddy VS, Raghu G, Reddy SS, Pasupulati AK, Suryanarayana P, Reddy GB. Response of small heat shock proteins in diabetic rat retina. Invest Ophthalmol Vis Sci 54: 7674–7682, 2013. [DOI] [PubMed] [Google Scholar]
- 484.Reine TM, Grondahl F, Jenssen TG, Hadler-Olsen E, Prydz K, Kolset SO. Reduced sulfation of chondroitin sulfate but not heparan sulfate in kidneys of diabetic db/db mice. J Histochem Cytochem 61: 606–616, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Reine TM, Lanzalaco F, Kristiansen O, Enget AR, Satchell S, Jenssen TG, Kolset SO. Matrix metalloproteinase-9 mediated shedding of syndecan-4 in glomerular endothelial cells. Microcirculation e12534, 2019. [DOI] [PubMed] [Google Scholar]
- 486.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454: 345–359, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Remington LA and Remington LA. Chapter 4 - Retina. In: Clinical Anatomy and Physiology of the Visual System (Third Edition), Saint Louis: Butterworth-Heinemann, 2012, p. 61–92. [Google Scholar]
- 488.Rho YH, Solus J, Raggi P, Oeser A, Gebretsadik T, Shintani A, Stein CM. Macrophage activation and coronary atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res (Hoboken) 63: 535–541, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Ricciotti E and FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31: 986–1000, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Rimmer T and Linsenmeier RA. Resistance of diabetic rat electroretinogram to hypoxemia. Invest Ophthalmol Vis Sci 34: 3246–3252, 1993. [PubMed] [Google Scholar]
- 491.Riva CE, Grunwald JE and Petrig BL. Autoregulation of human retinal blood flow. An investigation with laser Doppler velocimetry. Invest Ophthalmol Vis Sci 27: 1706–1712, 1986. [PubMed] [Google Scholar]
- 492.Riva CE, Grunwald JE and Petrig BL. Reactivity of the human retinal circulation to darkness: a laser Doppler velocimetry study. Invest Ophthalmol Vis Sci 24: 737–740, 1983. [PubMed] [Google Scholar]
- 493.Riva CE, Grunwald JE and Sinclair SH. Laser Doppler Velocimetry study of the effect of pure oxygen breathing on retinal blood flow. Invest Ophthalmol Vis Sci 24: 47–51, 1983. [PubMed] [Google Scholar]
- 494.Riva CE, Logean E and Falsini B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Retin Eye Res 24: 183–215, 2005. [DOI] [PubMed] [Google Scholar]
- 495.Riva CE, Logean E, Petrig BL, Falsini B. [Effect of dark adaptation on retinal blood flow]. Klinische Monatsblatter fur Augenheilkunde 216: 309–310, 2000. [DOI] [PubMed] [Google Scholar]
- 496.Riva CE, Pournaras CJ and Tsacopoulos M. Regulation of local oxygen tension and blood flow in the inner retina during hyperoxia. J Appl Physiol (1985) 61: 592–598, 1986. [DOI] [PubMed] [Google Scholar]
- 497.Riva CE, Sinclair SH and Grunwald JE. Autoregulation of retinal circulation in response to decrease of perfusion pressure. Invest Ophthalmol Vis Sci 21: 34–38, 1981. [PubMed] [Google Scholar]
- 498.Rival Y, Del Maschio A, Rabiet MJ, Dejana E, Duperray A. Inhibition of platelet endothelial cell adhesion molecule-1 synthesis and leukocyte transmigration in endothelial cells by the combined action of TNF-alpha and IFN-gamma. J Immunol 157: 1233–1241, 1996. [PubMed] [Google Scholar]
- 499.Robertson PL, Ar D and Goldstein GW. Phosphoinositide metabolism and prostacyclin formation in retinal microvascular endothelium: stimulation by adenine nucleotides. Experimental eye research 50: 37–44, 1990. [DOI] [PubMed] [Google Scholar]
- 500.Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci 27: 722–726, 1986. [PubMed] [Google Scholar]
- 501.Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol 154: 6582–6592, 1995. [PubMed] [Google Scholar]
- 502.Roy S and Amin S. Retinal fibrosis in diabetic retinopathy. Experimental eye research 142: 71–75, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Ruiz-Loredo AY and López-Colomé AM. Chapter three - New Insights into the Regulation of Myosin Light Chain Phosphorylation in Retinal Pigment Epithelial Cells. In: International Review of Cell and Molecular Biology, edited by Jeon KW. Academic Press, 2012, p. 85–121. [DOI] [PubMed] [Google Scholar]
- 504.Ryan SJ, Puliafito CA, Davis JL, Parel J-M, Milne P, Ryan S, Moshiri A. Retina. Philadelphia: Elsevier Mosby, 2006, p. 2731. [Google Scholar]
- 505.Sachs UJ, Andrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT, Chavakis T, Santoso S. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31). The Journal of biological chemistry 282: 23603–23612, 2007. [DOI] [PubMed] [Google Scholar]
- 506.Samra YA, Saleh HM, Hussein KA, Elsherbiny NM, Ibrahim AS, Elmasry K, Fulzele S, El-Shishtawy MM, Eissa LA, Al-Shabrawey M, Liou GI. Adenosine Deaminase-2-Induced Hyperpermeability in Human Retinal Vascular Endothelial Cells Is Suppressed by MicroRNA-146b-3p. Invest Ophthalmol Vis Sci 58: 933–943, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Sanes JR and Masland RH. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci 38: 221–246, 2015. [DOI] [PubMed] [Google Scholar]
- 508.Santos KG, Crispim D, Canani LH, Ferrugem PT, Gross JL, Roisenberg I. Relationship of endothelial nitric oxide synthase (eNOS) gene polymorphisms with diabetic retinopathy in Caucasians with type 2 diabetes. Ophthalmic Genet 33: 23–27, 2012. [DOI] [PubMed] [Google Scholar]
- 509.Sarlos S, Rizkalla B, Moravski CJ, Cao Z, Cooper ME, Wilkinson-Berka JL. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. The American journal of pathology 163: 879–887, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Savage HI, Hendrix JW, Peterson DC, Young H, Wilkinson CP. Differences in pulsatile ocular blood flow among three classifications of diabetic retinopathy. Invest Ophthalmol Vis Sci 45: 4504–4509, 2004. [DOI] [PubMed] [Google Scholar]
- 511.Sawa Y, Sugimoto Y, Ueki T, Ishikawa H, Sato A, Nagato T, Yoshida S. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochem Cytochem 55: 721–733, 2007. [DOI] [PubMed] [Google Scholar]
- 512.Schaefer S, Kajimura M, Tsuyama S, Uchida K, Sato E, Inoue M, Suematsu M, Watanabe K. Aberrant utilization of nitric oxide and regulation of soluble guanylate cyclase in rat diabetic retinopathy. Antioxid Redox Signal 5: 457–465, 2003. [DOI] [PubMed] [Google Scholar]
- 513.Schafer M, Schafer C, Ewald N, Piper HM, Noll T. Role of redox signaling in the autonomous proliferative response of endothelial cells to hypoxia. Circ Res 92: 1010–1015, 2003. [DOI] [PubMed] [Google Scholar]
- 514.Schenkel AR, Chew TW and Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. In: J Immunol, United States: 2004, vol. 10, p. 6403–6408. [DOI] [PubMed] [Google Scholar]
- 515.Schmetterer L, Findl O, Fasching P, Ferber W, Strenn K, Breiteneder H, Adam H, Eichler HG, Wolzt M. Nitric oxide and ocular blood flow in patients with IDDM. Diabetes 46: 653–658, 1997. [DOI] [PubMed] [Google Scholar]
- 516.Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL, Stern D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb 14: 1521–1528, 1994. [DOI] [PubMed] [Google Scholar]
- 517.Schmidt KG, von Ruckmann A, Kemkes-Matthes B, Hammes HP. Ocular pulse amplitude in diabetes mellitus. Br J Ophthalmol 84: 1282–1284, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Schneider MP, Boesen EI and Pollock DM. Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol 47: 731–759, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Schocket LS, Brucker AJ, Niknam RM, Grunwald JE, DuPont J, Brucker AJ. Foveolar choroidal hemodynamics in proliferative diabetic retinopathy. International ophthalmology 25: 89–94, 2004. [DOI] [PubMed] [Google Scholar]
- 520.Schreiber V. [EDRF-NO. The endothelium-derived relaxing factor is nitric oxide]. Cas Lek Cesk 131: 388–390, 1992. [PubMed] [Google Scholar]
- 521.Schroder S, Palinski W and Schmid-Schonbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. The American journal of pathology 139: 81–100, 1991. [PMC free article] [PubMed] [Google Scholar]
- 522.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 88: 1474–1480, 2000. [DOI] [PubMed] [Google Scholar]
- 523.Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J Diabetes Res 2015: 582060, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Sena CM, Pereira AM and Seiça R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta 1832: 2216–2231, 2013. [DOI] [PubMed] [Google Scholar]
- 525.Shafat I, Ilan N, Zoabi S, Vlodavsky I, Nakhoul F. Heparanase levels are elevated in the urine and plasma of type 2 diabetes patients and associate with blood glucose levels. PLoS One 6: e17312, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Sharma M, Jamieson C, Johnson M, Molloy MP, Henderson BR. Specific armadillo repeat sequences facilitate β-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. The Journal of biological chemistry 287: 819–831, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 527.Sharma S, Saxena S, Srivastav K, Shukla RK, Mishra N, Meyer CH, Kruzliak P, Khanna VK. Nitric oxide and oxidative stress is associated with severity of diabetic retinopathy and retinal structural alterations. Clin Exp Ophthalmol 43: 429–436, 2015. [DOI] [PubMed] [Google Scholar]
- 528.Sharma SD, Pandey BN, Mishra KP, Sivakami S. Amadori product and age formation during nonenzymatic glycosylation of bovine serum albumin in vitro. J Biochem Mol Biol Biophys 6: 233–242, 2002. [DOI] [PubMed] [Google Scholar]
- 529.Shaw SK, Perkins BN, Lim YC, Liu Y, Nusrat A, Schnell FJ, Parkos CA, Luscinskas FW. Reduced Expression of Junctional Adhesion Molecule and Platelet/Endothelial Cell Adhesion Molecule-1 (CD31) at Human Vascular Endothelial Junctions by Cytokines Tumor Necrosis Factor-α Plus Interferon-γ Does Not Reduce Leukocyte Transmigration Under Flow. In: The American journal of pathology: 2001, vol. 6, p. 2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Sheikpranbabu S, Haribalaganesh R and Gurunathan S. Pigment epithelium-derived factor inhibits advanced glycation end-products-induced cytotoxicity in retinal pericytes. Diabetes Metab 37: 505–511, 2011. [DOI] [PubMed] [Google Scholar]
- 531.Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. The American journal of physiology 265: E783–793, 1993. [DOI] [PubMed] [Google Scholar]
- 532.Shitashige M, Satow R, Honda K, Ono M, Hirohashi S, Yamada T. Regulation of Wnt signaling by the nuclear pore complex. Gastroenterology 134: 1961–1971, 1971.e1961-1964, 2008. [DOI] [PubMed] [Google Scholar]
- 533.Shu J and Santulli G. Heparanase in health and disease: The neglected housekeeper of the cell? Atherosclerosis 283: 124–126, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Simmons RA, Polin RA, Abman SH, Rowitch DH, Benitz WE, Fox WW. 43 - Cell Glucose Transport and Glucose Handling During Fetal and Neonatal Development. In: Fetal and Neonatal Physiology (Fifth Edition): Elsevier, 2017, p. 428–435.e423. [Google Scholar]
- 535.Sinclair SH, Grunwald JE, Riva CE, Braunstein SN, Nichols CW, Schwartz SS. Retinal vascular autoregulation in diabetes mellitus. Ophthalmology 89: 748–750, 1982. [DOI] [PubMed] [Google Scholar]
- 536.Singh A, Friden V, Dasgupta I, Foster RR, Welsh GI, Tooke JE, Haraldsson B, Mathieson PW, Satchell SC. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Renal Physiol 300: F40–48, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 18: 1–14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Sjolie AK, Dodson P and Hobbs FR. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. International journal of clinical practice 65: 148–153, 2011. [DOI] [PubMed] [Google Scholar]
- 539.Small KW, Stefansson E and Hatchell DL. Retinal blood flow in normal and diabetic dogs. Invest Ophthalmol Vis Sci 28: 672–675, 1987. [PubMed] [Google Scholar]
- 540.Smith RS, John SWM and Nishina PM. The Posterior Segment and Orbit. In: Systematic Evaluation of the Mouse Eye: Anatomy, Pathology, and Biomethods, edited by John SWM, Nishina PM and Sundberg JP. Baco Raton: CRC Press LLC: Taylor & Francis Group, 2002, p. 25–44. [Google Scholar]
- 541.Smith RS and Rudt LA. Ocular vascular and epithelial barriers to microperoxidase. Investigative ophthalmology 14: 556–560, 1975. [PubMed] [Google Scholar]
- 542.Sogawa K, Nagaoka T, Izumi N, Nakabayashi S, Yoshida A. Acute hyperglycemia-induced endothelial dysfunction in retinal arterioles in cats. Invest Ophthalmol Vis Sci 51: 2648–2655, 2010. [DOI] [PubMed] [Google Scholar]
- 543.Someya E, Akagawa M, Mori A, Morita A, Yui N, Asano D, Sakamoto K, Nakahara T. Role of Neuron–Glia Signaling in Regulation of Retinal Vascular Tone in Rats. International journal of molecular sciences 20: 1952, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Sone H, Deo BK and Kumagai AK. Enhancement of glucose transport by vascular endothelial growth factor in retinal endothelial cells. Invest Ophthalmol Vis Sci 41: 1876–1884, 2000. [PubMed] [Google Scholar]
- 545.Song HB, Jun HO, Kim JH, Yu YS, Kim KW. Suppression of protein kinase C-ζ attenuates vascular leakage via prevention of tight junction protein decrease in diabetic retinopathy. Biochemical and biophysical research communications 444: 63–68, 2014. [DOI] [PubMed] [Google Scholar]
- 546.Song Y, Nagaoka T, Yoshioka T, Nakabayashi S, Tani T, Yoshida A. Role of Glial Cells in Regulating Retinal Blood Flow During Flicker-Induced Hyperemia in Cats. Invest Ophthalmol Vis Sci 56: 7551–7559, 2015. [DOI] [PubMed] [Google Scholar]
- 547.Sorrentino FS, Matteini S, Bonifazzi C, Sebastiani A, Parmeggiani F. Diabetic retinopathy and endothelin system: microangiopathy versus endothelial dysfunction. Eye (London, England) 32: 1157–1163, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Starr CR and Engleberg NC. Role of hyaluronidase in subcutaneous spread and growth of group A streptococcus. Infect Immun 74: 40–48, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Starr MS. Effects of prostaglandin on blood flow in the rabbit eye. Experimental eye research 11: 161–169, 1971. [DOI] [PubMed] [Google Scholar]
- 550.Stefansson E, Hatchell DL, Fisher BL, Sutherland FS, Machemer R. Panretinal photocoagulation and retinal oxygenation in normal and diabetic cats. American journal of ophthalmology 101: 657–664, 1986. [DOI] [PubMed] [Google Scholar]
- 551.Stefansson E, Peterson JI and Wang YH. Intraocular oxygen tension measured with a fiber-optic sensor in normal and diabetic dogs. The American journal of physiology 256: H1127–1133, 1989. [DOI] [PubMed] [Google Scholar]
- 552.Stefansson E, Wagner HG and Seida M. Retinal blood flow and its autoregulation measured by intraocular hydrogen clearance. Experimental eye research 47: 669–678, 1988. [DOI] [PubMed] [Google Scholar]
- 553.Stepniak E, Radice GL and Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol 1: a002949, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Stewart RJ, Kashour TS and Marsden PA. Vascular endothelial platelet endothelial adhesion molecule-1 (PECAM-1) expression is decreased by TNF-alpha and IFN-gamma. Evidence for cytokine-induced destabilization of messenger ribonucleic acid transcripts in bovine endothelial cells. J Immunol 156: 1221–1228, 1996. [PubMed] [Google Scholar]
- 555.Stjernschantz J, Alm A and Bill A. Effects of intracranial oculomotor nerve stimulation on ocular blood flow in rabbits: modification by indomethacin. Experimental eye research 23: 461–469, 1976. [DOI] [PubMed] [Google Scholar]
- 556.Stjernschantz J and Bill A. Vasomotor effects of facial nerve stimulation: noncholinergic vasodilation in the eye. Acta physiologica Scandinavica 109: 45–50, 1980. [DOI] [PubMed] [Google Scholar]
- 557.Stjernschantz J, Geijer C and Bill A. Electrical stimulation of the fifth cranial nerve in rabbits: effects on ocular blood flow, extravascular albumin content and intraocular pressure. Experimental eye research 28: 229–238, 1979. [DOI] [PubMed] [Google Scholar]
- 558.Su EN, Alder VA, Yu DY, Yu PK, Cringle SJ, Yogesan K. Continued progression of retinopathy despite spontaneous recovery to normoglycemia in a long-term study of streptozotocin-induced diabetes in rats. Graefes Arch Clin Exp Ophthalmol 238: 163–173, 2000. [DOI] [PubMed] [Google Scholar]
- 559.Su J, An XR, Li Q, Li XX, Cong XD, Xu M. Improvement of vascular dysfunction by argirein through inhibiting endothelial cell apoptosis associated with ET-1/Nox4 signal pathway in diabetic rats. Sci Rep 8: 12620, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Suarez G, Rajaram R, Oronsky AL, Gawinowicz MA. Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. The Journal of biological chemistry 264: 3674–3679, 1989. [PubMed] [Google Scholar]
- 561.Sullivan DP, Rüffer C and Muller WA. Isolation of the lateral border recycling compartment using a diaminobenzidine-induced density shift. Traffic 15: 1016–1029, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Sutherland FS, Stefansson E, Hatchell DL, Reiser H. Retinal oxygen consumption in vitro. The effect of diabetes mellitus, oxygen and glucose. Acta Ophthalmol (Copenh) 68: 715–720, 1990. [DOI] [PubMed] [Google Scholar]
- 563.Takagi C, Bursell SE, Lin YW, Takagi H, Duh E, Jiang Z, Clermont AC, King GL. Regulation of retinal hemodynamics in diabetic rats by increased expression and action of endothelin-1. Invest Ophthalmol Vis Sci 37: 2504–2518, 1996. [PubMed] [Google Scholar]
- 564.Takagi C, King GL, Clermont AC, Cummins DR, Takagi H, Bursell SE. Reversal of abnormal retinal hemodynamics in diabetic rats by acarbose, an alpha-glucosidase inhibitor. Current eye research 14: 741–749, 1995. [DOI] [PubMed] [Google Scholar]
- 565.Takagi H, King GL and Aiello LP. Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes 47: 1480–1488, 1998. [DOI] [PubMed] [Google Scholar]
- 566.Takei K, Sato T, Nonoyama T, Miyauchi T, Goto K, Hommura S. A new model of transient complete obstruction of retinal vessels induced by endothelin-1 injection into the posterior vitreous body in rabbits. Graefes Arch Clin Exp Ophthalmol 231: 476–481, 1993. [DOI] [PubMed] [Google Scholar]
- 567.Tang J and Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res 30: 343–358, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol 2013: 343560, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Tattersall. Diabetes: The Biography. Oxford University Press, 2009, p. 240. [Google Scholar]
- 570.Taylor E and Dobree JH. Proliferative diabetic retinopathy. Site and size of initial lesions. Br J Ophthalmol 54: 11–18, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Testa R, Bonfigli AR, Prattichizzo F, La Sala L, De Nigris V, Ceriello A. The "Metabolic Memory" Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 9: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Thomas B and Caballero B. DIABETES MELLITUS ∣ Secondary Complications. In: Encyclopedia of Food Sciences and Nutrition (Second Edition), Oxford: Academic Press, 2003, p. 1800–1804. [Google Scholar]
- 573.Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A, McSorley KM, Renis RL, Kern T, Jump DB, Reid GE, Busik JV. Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes 59: 219–227, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Tilton RG, Chang K, Pugliese G, Eades DM, Province MA, Sherman WR, Kilo C, Williamson JR. Prevention of hemodynamic and vascular albumin filtration changes in diabetic rats by aldose reductase inhibitors. Diabetes 38: 1258–1270, 1989. [DOI] [PubMed] [Google Scholar]
- 575.Titchenell PM, Lin CM, Keil JM, Sundstrom JM, Smith CD, Antonetti DA. Novel atypical PKC inhibitors prevent vascular endothelial growth factor-induced blood-retinal barrier dysfunction. The Biochemical journal 446: 455–467, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Toda N, Kitamura Y and Okamura T. Role of nitroxidergic nerve in dog retinal arterioles in vivo and arteries in vitro. The American journal of physiology 266: H1985–1992, 1994. [DOI] [PubMed] [Google Scholar]
- 577.Tonade D, Liu H and Kern TS. Photoreceptor Cells Produce Inflammatory Mediators That Contribute to Endothelial Cell Death in Diabetes. Invest Ophthalmol Vis Sci 57: 4264–4271, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Tornquist P and Alm A. Retinal and choroidal contribution to retinal metabolism in vivo. A study in pigs. Acta physiologica Scandinavica 106: 351–357, 1979. [DOI] [PubMed] [Google Scholar]
- 579.Trick GL and Berkowtiz BA. Retinal oxygenation response and retinopathy. Progress in retinal and eye research 24: 259–274, 2005. [DOI] [PubMed] [Google Scholar]
- 580.Trudeau K, Molina AJ, Guo W, Roy S. High Glucose Disrupts Mitochondrial Morphology in Retinal Endothelial Cells : Implications for Diabetic Retinopathy. The American journal of pathology 177: 447–455, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Tsacopoulos M, Baker R, Johnson M, Strauss J, David NJ. The effect of arterial PCO2 on inner-retinal oxygen availability in monkeys. Investigative ophthalmology 12: 449–455, 1973. [PubMed] [Google Scholar]
- 582.Tsacopoulos M and David NJ. The effect of arterial PCO 2 on relative retinal blood flow in monkeys. Investigative ophthalmology 12: 335–347, 1973. [PubMed] [Google Scholar]
- 583.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005. [DOI] [PubMed] [Google Scholar]
- 584.Ullrich V, Zou MH and Bachschmid M. New physiological and pathophysiological aspects on the thromboxane A(2)-prostacyclin regulatory system. Biochim Biophys Acta 1532: 1–14, 2001. [DOI] [PubMed] [Google Scholar]
- 585.van den Hoven MJ, Rops AL, Bakker MA, Aten J, Rutjes N, Roestenberg P, Goldschmeding R, Zcharia E, Vlodavsky I, van der Vlag J, Berden JH. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int 70: 2100–2108, 2006. [DOI] [PubMed] [Google Scholar]
- 586.van Dijk HW, Kok PH, Garvin M, Sonka M, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO, Verbraak FD, Abramoff MD. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci 50: 3404–3409, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Van Geest RJ, Leeuwis JW, Dendooven A, Pfister F, Bosch K, Hoeben KA, Vogels IM, Van der Giezen DM, Dietrich N, Hammes HP, Goldschmeding R, Klaassen I, Van Noorden CJ, Schlingemann RO. Connective tissue growth factor is involved in structural retinal vascular changes in long-term experimental diabetes. J Histochem Cytochem 62: 109–118, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Vanhoutte PM, Rubanyi GM, Miller VM, Houston DS. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol 48: 307–320, 1986. [DOI] [PubMed] [Google Scholar]
- 589.Venkataraman ST, Hudson C, Fisher JA, Flanagan JG. Novel methodology to comprehensively assess retinal arteriolar vascular reactivity to hypercapnia. Microvasc Res 72: 101–107, 2006. [DOI] [PubMed] [Google Scholar]
- 590.Venturini CM, Knowles RG, Palmer RM, Moncada S. Synthesis of nitric oxide in the bovine retina. Biochemical and biophysical research communications 180: 920–925, 1991. [DOI] [PubMed] [Google Scholar]
- 591.Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia 58: 886–899, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Vials A and Burnstock G. A2-purinoceptor-mediated relaxation in the guinea-pig coronary vasculature: a role for nitric oxide. British journal of pharmacology 109: 424–429, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Vink H and Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 79: 581–589, 1996. [DOI] [PubMed] [Google Scholar]
- 594.Vinores SA, Van Niel E, Swerdloff JL, Campochiaro PA. Electron microscopic immunocytochemical demonstration of blood-retinal barrier breakdown in human diabetics and its association with aldose reductase in retinal vascular endothelium and retinal pigment epithelium. Histochem J 25: 648–663, 1993. [DOI] [PubMed] [Google Scholar]
- 595.Vogl-Willis CA and Edwards IJ. High-glucose-induced structural changes in the heparan sulfate proteoglycan, perlecan, of cultured human aortic endothelial cells. Biochim Biophys Acta 1672: 36–45, 2004. [DOI] [PubMed] [Google Scholar]
- 596.Wagner JD, Cann JA, Zhang L, Harwood HJ, Abee CR, Mansfield K, Tardif S, Morris T. Chapter 14 - Diabetes and Obesity Research using Nonhuman Primates. In: Nonhuman Primates in Biomedical Research (Second Edition), Boston: Academic Press, 2012, p. 699–732. [Google Scholar]
- 597.Wakabayashi Y, Usui Y, Okunuki Y, Takeuchi M, Kezuka T, Iwasaki T, Goto H. Increased levels of monokine induced by interferon-gamma (Mig) in the vitreous of patients with diabetic retinopathy. Diabet Med 25: 875–877, 2008. [DOI] [PubMed] [Google Scholar]
- 598.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol 17: 331–367, 1999. [DOI] [PubMed] [Google Scholar]
- 599.Wanek J, Teng PY, Blair NP, Shahidi M. Inner retinal oxygen delivery and metabolism in streptozotocin diabetic rats. Invest Ophthalmol Vis Sci 55: 1588–1593, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, Gutterman DD, Widlansky ME. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 32: 712–720, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Wang L, Fortune B, Cull G, McElwain KM, Cioffi GA. Microspheres method for ocular blood flow measurement in rats: size and dose optimization. Experimental eye research 84: 108–117, 2007. [DOI] [PubMed] [Google Scholar]
- 602.Wang L, Grant C, Fortune B, Cioffi GA. Retinal and choroidal vasoreactivity to altered PaCO2 in rat measured with a modified microsphere technique. Experimental eye research 86: 908–913, 2008. [DOI] [PubMed] [Google Scholar]
- 603.Wang S, Villegas-Perez MP, Vidal-Sanz M, Lund RD. Progressive optic axon dystrophy and vacuslar changes in rd mice. Invest Ophthalmol Vis Sci 41: 537–545, 2000. [PubMed] [Google Scholar]
- 604.Wang W, Sawicki G and Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res 53: 165–174, 2002. [DOI] [PubMed] [Google Scholar]
- 605.Wang Z, Yadav AS, Leskova W, Harris NR. Attenuation of streptozotocin-induced microvascular changes in the mouse retina with the endothelin receptor A antagonist atrasentan. Experimental eye research 91: 670–675, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Wang Z, Yadav AS, Leskova W, Harris NR. Inhibition of 20-HETE attenuates diabetes-induced decreases in retinal hemodynamics. Experimental eye research 93: 108–113, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Wangsa-Wirawan ND and Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 121: 547–557, 2003. [DOI] [PubMed] [Google Scholar]
- 608.Wardle EN. Vascular permeability in diabetics and implications for therapy. Diabetes Res Clin Pract 23: 135–139, 1994. [DOI] [PubMed] [Google Scholar]
- 609.Watanabe T, Matsushima S, Okazaki M, Nagamatsu S, Hirosawa K, Uchimura H, Nakahara K. Localization and ontogeny of GLUT3 expression in the rat retina. Brain research Developmental brain research 94: 60–66, 1996. [DOI] [PubMed] [Google Scholar]
- 610.Watt SM, Gschmeissner SE and Bates PA. PECAM-1: Its Expression and Function as a Cell Adhesion Molecule on Hemopoietic and Endothelial Cells. 2009. [DOI] [PubMed] [Google Scholar]
- 611.Wheeler-Schilling TH, Kohler K, Sautter M, Guenther E. Angiotensin II receptor subtype gene expression and cellular localization in the retina and non-neuronal ocular tissues of the rat. The European journal of neuroscience 11: 3387–3394, 1999. [DOI] [PubMed] [Google Scholar]
- 612.Wilkinson-Berka JL. Vasoactive factors and diabetic retinopathy: vascular endothelial growth factor, cycoloxygenase-2 and nitric oxide. Curr Pharm Des 10: 3331–3348, 2004. [DOI] [PubMed] [Google Scholar]
- 613.Williams B, Gallacher B, Patel H, Orme C. Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes 46: 1497–1503, 1997. [DOI] [PubMed] [Google Scholar]
- 614.Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, Nyengaard JR, van den Enden M, Kilo C, Tilton RG. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes 42: 801–813, 1993. [DOI] [PubMed] [Google Scholar]
- 615.Winkler BS, Starnes CA, Sauer MW, Firouzgan Z, Chen SC. Cultured retinal neuronal cells and Muller cells both show net production of lactate. Neurochemistry international 45: 311–320, 2004. [DOI] [PubMed] [Google Scholar]
- 616.Winkler JL, Kedees MH, Guz Y, Teitelman G. Inhibition of connective tissue growth factor by small interfering ribonucleic acid prevents increase in extracellular matrix molecules in a rodent model of diabetic retinopathy. 2012. [PMC free article] [PubMed] [Google Scholar]
- 617.Winn RK and Harlan JM. The role of endothelial cell apoptosis in inflammatory and immune diseases. J Thromb Haemost 3: 1815–1824, 2005. [DOI] [PubMed] [Google Scholar]
- 618.Wise GN. Retinal neovascularization. Trans Am Ophthalmol Soc 54: 729–826, 1956. [PMC free article] [PubMed] [Google Scholar]
- 619.Wisniewska-Kruk J, Klaassen I, Vogels IM, Magno AL, Lai CM, Van Noorden CJ, Schlingemann RO, Rakoczy EP. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Experimental eye research 122: 123–131, 2014. [DOI] [PubMed] [Google Scholar]
- 620.Woodfin A, Reichel CA, Khandoga A, Corada M, Voisin MB, Scheiermann C, Haskard DO, Dejana E, Krombach F, Nourshargh S. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood 110: 1848–1856, 2007. [DOI] [PubMed] [Google Scholar]
- 621.Woodfin A, Voisin MB and Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol 27: 2514–2523, 2007. [DOI] [PubMed] [Google Scholar]
- 622.Wright WS and Harris NR. Ozagrel attenuates early streptozotocin-induced constriction of arterioles in the mouse retina. Experimental eye research 86: 528–536, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Wright WS, McElhatten RM and Harris NR. Expression of thromboxane synthase and the thromboxane-prostanoid receptor in the mouse and rat retina. Experimental eye research 89: 532–537, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Wright WS, McElhatten RM and Harris NR. Increase in retinal hypoxia-inducible factor-2alpha, but not hypoxia, early in the progression of diabetes in the rat. Experimental eye research 93: 437–441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Wright WS, McElhatten RM, Messina JE, Harris NR. Hypoxia and the expression of HIF-1alpha and HIF-2alpha in the retina of streptozotocin-injected mice and rats. Experimental eye research 90: 405–412, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Wright WS, Messina JE and Harris NR. Attenuation of diabetes-induced retinal vasoconstriction by a thromboxane receptor antagonist. Experimental eye research 88: 106–112, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Wright WS, Yadav AS, McElhatten RM, Harris NR. Retinal blood flow abnormalities following six months of hyperglycemia in the Ins2(Akita) mouse. Experimental eye research 98: 9–15, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Wu D, Peng F, Zhang B, Ingram AJ, Kelly DJ, Gilbert RE, Gao B, Krepinsky JC. PKC-beta1 mediates glucose-induced Akt activation and TGF-beta1 upregulation in mesangial cells. J Am Soc Nephrol 20: 554–566, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes 43: 1122–1129, 1994. [DOI] [PubMed] [Google Scholar]
- 630.Xu Z, Tong C and Eisenach JC. Acetylcholine stimulates the release of nitric oxide from rat spinal cord. Anesthesiology 85: 107–111, 1996. [DOI] [PubMed] [Google Scholar]
- 631.Yadav AS and Harris NR. Effect of tempol on diabetes-induced decreases in retinal blood flow in the mouse. Current eye research 36: 456–461, 2011. [DOI] [PubMed] [Google Scholar]
- 632.Yam JC and Kwok AK. Update on the treatment of diabetic retinopathy. Hong Kong Med J 13: 46–60, 2007. [PubMed] [Google Scholar]
- 633.Yamamoto R, Bredt DS, Snyder SH, Stone RA. The localization of nitric oxide synthase in the rat eye and related cranial ganglia. Neuroscience 54: 189–200, 1993. [DOI] [PubMed] [Google Scholar]
- 634.Yamanishi S, Katsumura K, Kobayashi T, Puro DG. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. American journal of physiology Heart and circulatory physiology 290: H925–934, 2006. [DOI] [PubMed] [Google Scholar]
- 635.Yamashiro K, Tsujikawa A, Ishida S, Usui T, Kaji Y, Honda Y, Ogura Y, Adamis AP. Platelets accumulate in the diabetic retinal vasculature following endothelial death and suppress blood-retinal barrier breakdown. The American journal of pathology 163: 253–259, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988. [DOI] [PubMed] [Google Scholar]
- 637.Yao K, Tschudi M, Flammer J, Lüscher TF. Endothelium-dependent regulation of vascular tone of the porcine ophthalmic artery. Invest Ophthalmol Vis Sci 32: 1791–1798, 1991. [PubMed] [Google Scholar]
- 638.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35: 556–564, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Ye XD, Laties AM and Stone RA. Peptidergic innervation of the retinal vasculature and optic nerve head. Invest Ophthalmol Vis Sci 31: 1731–1737, 1990. [PubMed] [Google Scholar]
- 640.Yeagle PL and Yeagle PL. Chapter 13 - Membrane Transport. In: The Membranes of Cells (Third Edition), Boston: Academic Press, 2016, p. 335–378. [Google Scholar]
- 641.Yoshida S, Yoshida A and Ishibashi T. Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefes Arch Clin Exp Ophthalmol 242: 409–413, 2004. [DOI] [PubMed] [Google Scholar]
- 642.Yoshioka T, Nagaoka T, Song Y, Yokota H, Tani T, Yoshida A. Role of neuronal nitric oxide synthase in regulating retinal blood flow during flicker-induced hyperemia in cats. Invest Ophthalmol Vis Sci 56: 3113–3120, 2015. [DOI] [PubMed] [Google Scholar]
- 643.Yu DY and Cringle SJ. Outer retinal anoxia during dark adaptation is not a general property of mammalian retinas. Comp Biochem Physiol A Mol Integr Physiol 132: 47–52, 2002. [DOI] [PubMed] [Google Scholar]
- 644.Yu DY and Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res 20: 175–208, 2001. [DOI] [PubMed] [Google Scholar]
- 645.Yu DY and Cringle SJ. Oxygen distribution in the mouse retina. Invest Ophthalmol Vis Sci 47: 1109–1112, 2006. [DOI] [PubMed] [Google Scholar]
- 646.Yu DY, Cringle SJ, Alder VA, Su EN. Intraretinal oxygen distribution in rats as a function of systemic blood pressure. The American journal of physiology 267: H2498–2507, 1994. [DOI] [PubMed] [Google Scholar]
- 647.Yu DY, Cringle SJ, Alder VA, Su EN, Yu PK. Intraretinal oxygen distribution and choroidal regulation in the avascular retina of guinea pigs. The American journal of physiology 270: H965–973, 1996. [DOI] [PubMed] [Google Scholar]
- 648.Yu DY, Cringle SJ, Yu PK, Su EN. Intraretinal oxygen distribution and consumption during retinal artery occlusion and graded hyperoxic ventilation in the rat. Invest Ophthalmol Vis Sci 48: 2290–2296, 2007. [DOI] [PubMed] [Google Scholar]
- 649.Zhang J, Wu Y, Jin Y, Ji F, Sinclair SH, Luo Y, Xu G, Lu L, Dai W, Yanoff M, Li W, Xu GT. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci 49: 732–742, 2008. [DOI] [PubMed] [Google Scholar]
- 650.Zhang K, Ferreyra HA, Grob S, Bedell M, Zhang JJ, Ryan SJ, Sadda SR, Hinton DR, Schachat AP, Wilkinson CP, Wiedemann P. Chapter 46 - Diabetic Retinopathy: Genetics and Etiologic Mechanisms. In: Retina (Fifth Edition), London: W.B. Saunders, 2013, p. 925–939. [Google Scholar]
- 651.Zhang W, Chen S, Zhang Z, Wang C, Liu C. FAM3B mediates high glucose-induced vascular smooth muscle cell proliferation and migration via inhibition of miR-322-5p. Sci Rep 7: 2298, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Zhang W and Yan H. Simvastatin increases circulating endothelial progenitor cells and reduces the formation and progression of diabetic retinopathy in rats. Experimental eye research 105: 1–8, 2012. [DOI] [PubMed] [Google Scholar]
- 653.Zhao S, Li T, Zheng B, Zheng Z. Nitric oxide synthase 3 (NOS3) 4b/a, T-786C and G894T polymorphisms in association with diabetic retinopathy susceptibility: a meta-analysis. Ophthalmic Genet 33: 200–207, 2012. [DOI] [PubMed] [Google Scholar]
- 654.Zhao Y, Liu J, Ten S, Zhang J, Yuan Y, Yu J, An X. Plasma heparanase is associated with blood glucose levels but not urinary microalbumin excretion in type 2 diabetic nephropathy at the early stage. Ren Fail 39: 698–701, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Zheng L, Du Y, Miller C, Gubitosi-Klug R, Ball S, Berkowitz BA, Kern TS. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 50: 1987–1996, 2007. [DOI] [PubMed] [Google Scholar]
- 656.Zheng L, Szabo C and Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes 53: 2960–2967, 2004. [DOI] [PubMed] [Google Scholar]
- 657.Zhu Y, Park TS and Gidday JM. Mechanisms of hyperoxia-induced reductions in retinal blood flow in newborn pig. Experimental eye research 67: 357–369, 1998. [DOI] [PubMed] [Google Scholar]