Figure 1.
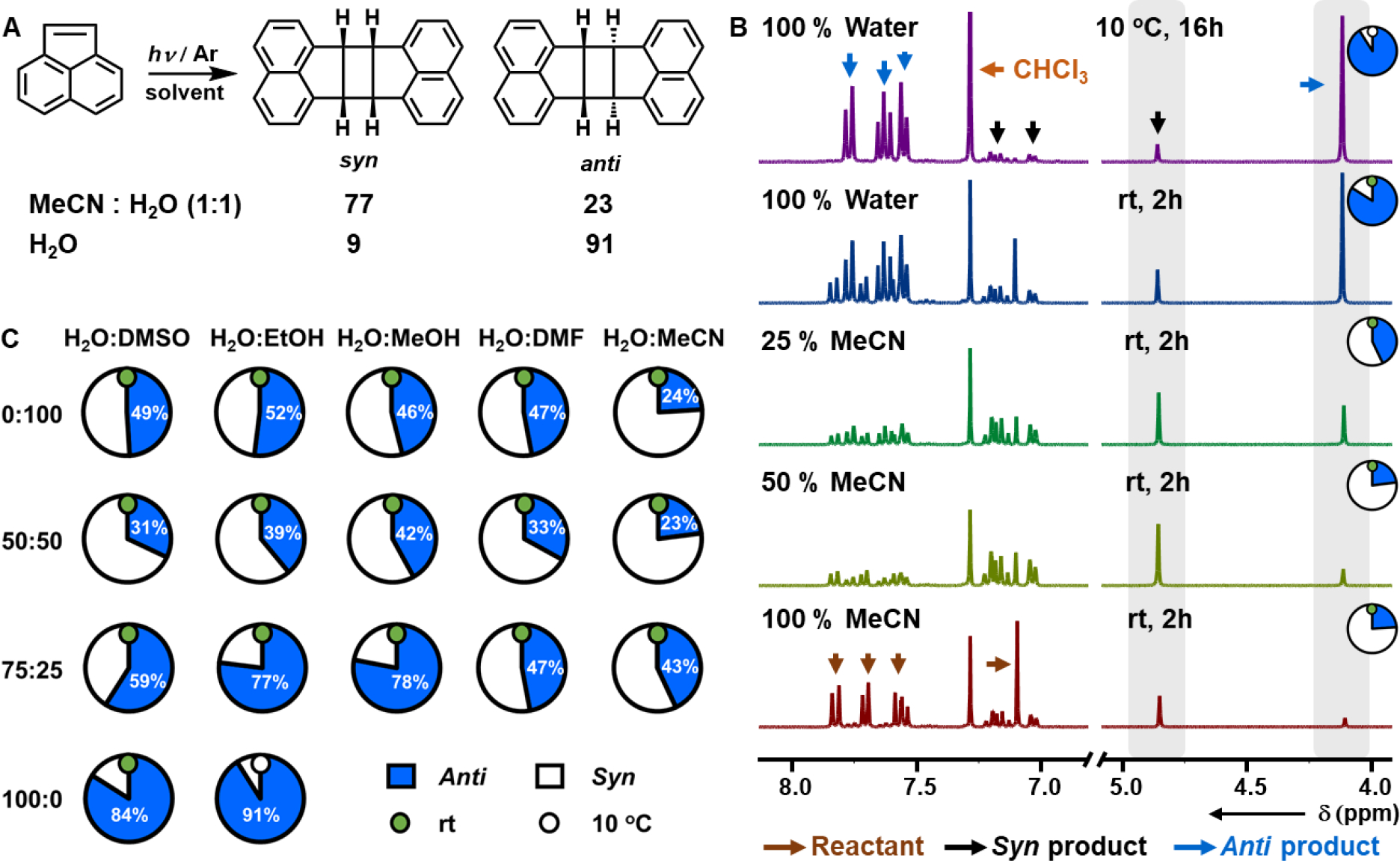
(A) [2+2] Photocycloaddition of acenaphthylene, with the most stereoselective conditions for syn and anti dimers noted. (B) 1H NMR (300 MHz, CDCl3) of the products of the [2+2] photocycloaddition of acenaphthylene in a binary solvent mixture composed of different ratios of MeCN and H2O. (C) Change in selectivity with changing solvent composition for the [2+2] photocycloaddition of acenaphthylene in binary solvent mixtures.
