Table 1.
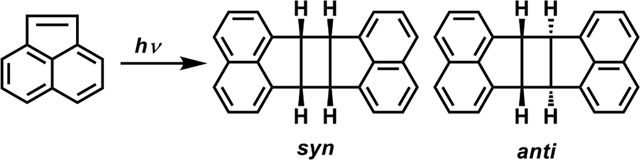
Solid-state [2+2] photocycloaddition of acenaphthylene.
| |||
|---|---|---|---|
|
| |||
| Entry | Atm. | Total Product Yield (%) |
Anti : Syn |
|
| |||
| 1 a | Ar | 95 | 6:94 |
| 2 a | air | 87 | 8:92 |
| 3 b | Ar | 65 | 70:30 |
| 4 b | air | 99 | 46:54 |
All reactions were performed at 20 °C. Acenaphthylene samples were irradiated with a blue LED (HepatoChem, DX Series light 30 W, λmax = 450 nm) for 20 h. Yields and selectivities were obtained from 1H NMR in CDCl3.
Ball-mill reactions performed in the presence of silica as an additive to prevent the reagents from adhering to the side walls of the reaction vessel.
Reactions were performed in a petri dish with ground acenaphthylene crystals and no milling during illumination.
