Abstract
Objectives. To describe participant characteristics associated with severe acute respiratory syndrome coronavirus 2 infection in Spain’s first 2 COVID-19 waves per the Spanish National Seroepidemiological Survey of SARS-CoV-2 Infection (ENE–COVID).
Methods. A representative cohort of the noninstitutionalized Spanish population, selected through stratified 2-stage sampling, answered a questionnaire and received point-of-care testing April to June 2020 (first wave: n = 68 287); previously seronegative participants repeated the questionnaire and test November 2020 (second wave: n = 44 451). We estimated seropositivity by wave and participant characteristics, accounting for sampling weights, nonresponse, and design effects.
Results. We found that 6.0% (95% confidence interval [CI] = 5.7%, 6.4%) of Spain’s population was infected by June and 3.8% (95% CI = 3.5%, 4.1%) more by November 2020. Both genders were equally affected. Seroprevalence decreased with age in adults 20 years and older in the second wave; socioeconomic differences increased. Health care workers were affected at 11.1% (95% CI = 9.0%, 13.6%) and 6.1% (95% CI = 4.4%, 8.5%) in the first and second waves, respectively. Living with an infected person increased infection risk to 22.1% (95% CI = 18.9%, 25.6%) in the first and 35.0% (95% CI = 30.8%, 39.4%) in the second wave.
Conclusions. ENE–COVID characterized the first 2 pandemic waves, when information from surveillance systems was incomplete. (Am J Public Health. 2023;113(5):533–544. https://doi.org/10.2105/AJPH.2023.307233)
When the World Health Organization declared the COVID-19 pandemic on March 11, 2020, Spain was among the most affected countries in Europe. On March 14, the Spanish government declared a state of emergency and a strict lockdown. Between April 28 and June 21, restrictions were progressively lifted, with different measures taken by each of the Spanish regions (known as autonomous communities).
Between January and April 2020, the National Epidemiological Surveillance System registered approximately 220 000 COVID-19 cases,1 an underestimate because of limited availability of diagnostic tests, which were reserved for those with severe illness and symptomatic health care workers. To more accurately estimate the number of infected people, the Spanish COVID-19 Task Force proposed a nationwide, population-based seroepidemiological study in late March, and this was launched a few weeks later, in April 2020. A cohort of individuals was invited to participate in 4 rounds of the study. The first 3 rounds, with the first starting on April 27, took place during the first pandemic wave. The fourth round took place in November 2020, after the peak of the second pandemic wave at the end of October.
We used data from the nationwide seroepidemiological study to describe participant characteristics associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the first 2 pandemic waves in Spain.
METHODS
The Spanish National Seroepidemiological Survey of SARS-CoV-2 Infection (Estudio Nacional de Sero-Epidemiología de la Infección por SARS-CoV-2 en España [ENE–COVID]) was a nationwide population-based cohort study to quantify the seropositivity for SARS-CoV-2 in the community-dwelling population of Spain.
Study Design and Population
We describe the study design in detail in our companion article (p. 525). Briefly, we selected 1500 census tracts and up to 24 households per tract through a 2-stage random sampling stratified by province and municipality size. We invited all residents in the 35 885 selected households to participate in the study. We collected serial data from epidemiological questionnaires and 2 serology tests (a point-of-care test and a laboratory immunoassay) from study participants in 2 survey phases. The first phase started 1 month after the peak incidence of the first COVID-19 pandemic wave in Spain and included 3 initial rounds of data collection at 3-week intervals between April 27 and June 22, 2020.
We conducted the second survey phase after the peak of the second pandemic wave and included a subsequent fourth round of data collection between November 16 and 30, 2020. Because of the comparable performance but easier implementation of the point-of-care test compared with the laboratory immunoassay during the first phase,2 the laboratory test was offered in the second survey phase only to participants from a random subcohort of 200 census tracts stratified by autonomous community (first-level territorial division integrating 1 or several provinces; Figure A of the Appendix, available as a supplement to the online version of this article at http://www.ajph.org).3
To study SARS-CoV-2 seroprevalence during the first pandemic wave, we used the data of 68 287 participants who received the point-of-care test and 61 095 who received the laboratory immunoassay in at least 1 of the 3 rounds of the first survey phase (response rates were 69.1% and 61.8% among all eligible persons, respectively). To investigate new seropositive participants for SARS-CoV-2 during the second pandemic wave, we used the data of 44 451 participants who tested negative by all received point-of-care tests during the first phase and underwent such a test in the single round of the second survey phase (retention rate was 69.2% among all seronegative participants in the first phase by the point-of-care test). In addition, we analyzed new seropositivity by laboratory immunoassay during the second pandemic wave in 5045 participants of the random subcohort who tested negative by the laboratory immunoassay in the first phase and received that test in the second survey phase (retention rate was 92.1% among subcohort participants with negative immunoassays in the first phase).
Data Collection and Serology Tests
Trained staff from the Spanish regional health services collected data using a common protocol developed by the National Centre for Epidemiology of the Instituto de Salud Carlos III and the Spanish Ministry of Health. At each survey round, residents in selected households were contacted by telephone and invited to go to their primary health care centers or to allow a home visit. Residents who agreed to participate answered an epidemiological questionnaire on sociodemographic characteristics, risk factors, comorbidities, disability, symptoms compatible with having COVID-19, polymerase chain reaction (PCR) status, and contact with someone with confirmed or suspected COVID-19. Participants also received a rapid serology test and, optionally, donated blood samples for further laboratory analysis. The survey protocol is detailed in the companion article (in this issue of AJPH; p. 525) and is available in Spanish on the ENE–COVID Web site3 and in a public repository (http://hdl.handle.net/20.500.12105/15247).
Briefly, the first serology test was a point-of-care rapid test applied to finger prick blood (COVID-19 IgG/IgM Rapid Test Cassette; Orient Gene Biotech, Zhejiang, China; reference GCCOV-402a) to detect the presence of immunoglobulin G (IgG) antibodies against SARS-CoV-2 spike protein; this showed a sensitivity of 82% and a specificity of 100% in a preliminary validation study.2 The second test was a chemiluminescent microparticle immunoassay (CMIA) using serum samples (SARS-CoV-2 IgG for use with ARCHITECT; Abbott Laboratories, Chicago, IL; reference 06R8620) to quantify IgG antibodies against the SARS-CoV-2 nucleoprotein. Using a threshold of 1.40 for the sample to calibrator chemiluminescent signal ratio, the CMIA showed a sensitivity of 91% and a specificity of 99% in a meta-analysis of 23 diagnostic accuracy studies.4
Statistical Analysis
We estimated seropositivity for SARS-CoV-2 during the first pandemic wave as the proportion of participants who had detectable IgG antibodies against SARS-CoV-2 in any round of the first survey phase by the point-of-care test. We estimated new seropositivity during the second pandemic wave as the proportion of seronegative participants in the first phase who had detectable IgG antibodies against SARS-CoV-2 in the single round of the second survey phase by the point-of-care test. We calculated new seropositive participants by pandemic wave in a similar way for the laboratory CMIA based on a seropositivity threshold of 1.40; results are presented as sensitivity analyses because of the lower response rate to the CMIA and its limited application to a subcohort in the second survey phase. For each pandemic wave, we calculated seropositivity ratios (SPRs) by participant characteristics, taking the overall seropositivity of that pandemic wave as the reference.
We performed further analyses combining results from both tests to maximize either sensitivity or specificity. We estimated seropositivity for SARS-CoV-2 during the first pandemic wave as the proportion of participants who were found positive by either test (most sensitive approach) or by both tests (most specific approach) in the first survey phase. We estimated new seropositivity status during the second pandemic wave as the proportion of seronegative participants as determined by both tests in the first phase who were found positive by either or both tests in the second survey phase.
We assigned sampling weights to survey participants to account for the different selection probabilities by province and autonomous community and to adjust for the distinct response rates to the point-of-care test and the CMIA by gender, age group, and census tract income category. We used different sampling weights for the point-of-care test and the CMIA in each pandemic wave, and we trimmed upper extreme weights (0.2% to 0.5%) to prevent influential observations. All statistical analyses accounted for the effect of stratification and clustering of seropositivity by household and census tract on SE estimates. We calculated confidence intervals (CIs) using logit-transformed seropositivity estimates and log-transformed ratios and back-transformed CIs to the original scale for reporting, with design-based degrees of freedom equal to the number of first-stage sampling units minus the number of strata. We performed analyses using survey commands in Stata, version 16 (StataCorp LP, College Station, TX).
RESULTS
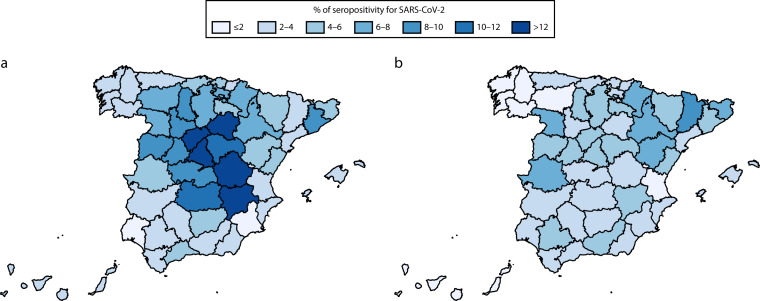
Figure 1 shows the geographical distribution of SARS-CoV-2 infection in the first and second pandemic waves. Table 1 presents seroprevalence figures together with seroprevalence ratios in each wave (Appendix Table A shows these figures separately in men and women). During the first pandemic wave, 6.0% of the Spanish population became infected. There were striking geographical differences (Figure 1), with seroprevalences greater than 10% concentrated in the middle of the country, whereas most of the coastal provinces had values lower than 4%. Table 1 shows seroprevalence figures and seroprevalence ratios according to different sociodemographic characteristics. Global prevalence figures were similar in both genders.
FIGURE 1—
New Seropositive Participants for SARS-CoV-2 as Determined by the Point-of-Care Test in the (a) First and (b) Second Pandemic Waves by Province: ENE–COVID, Spain, April 27–June 22 and November 16–30, 2020
Note. ENE–COVID = Spanish National Seroepidemiological Survey of SARS-CoV-2 Infection/Estudio Nacional de Sero-Epidemiología de la Infección por SARS-CoV-2 en España; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. The names of all Spanish provinces outlined on the maps are provided in Appendix Figure A (available as a supplement to the online version of this article at http://www.ajph.org).
TABLE 1—
New Seropositive Participants for SARS-CoV-2 as Determined by the Point-of-Care Test in the First and Second Pandemic Waves by General Characteristics: ENE–COVID, Spain, April 27–June 22 and November 16–30, 2020
| Characteristic | First Pandemic Wave | Second Pandemic Wave | ||||
| No. of Participantsa | Seropositivity,b % (95% CI) | Seropositivity Ratioc (95% CI) | No. of Participantsd | Seropositivity,e % (95% CI) | Seropositivity Ratioc (95% CI) | |
| Overall | 68 287 | 6.0 (5.7, 6.4) | 44 451 | 3.8 (3.5, 4.1) | ||
| Gender | ||||||
| Men | 32 742 | 5.9 (5.5, 6.4) | 0.98 (0.95, 1.02) | 20 956 | 3.7 (3.3, 4.1) | 0.98 (0.93, 1.04) |
| Women | 35 545 | 6.1 (5.8, 6.6) | 1.02 (0.98, 1.05) | 23 495 | 3.8 (3.5, 4.3) | 1.02 (0.97, 1.07) |
| Age, y | ||||||
| 0–19 | 12 581 | 4.0 (3.5, 4.5) | 0.66 (0.59, 0.74) | 7 543 | 4.0 (3.3, 4.8) | 1.06 (0.92, 1.21) |
| 20–34 | 9 552 | 5.1 (4.4, 5.8) | 0.84 (0.75, 0.95) | 5 217 | 4.3 (3.6, 5.1) | 1.14 (0.98, 1.32) |
| 35–49 | 16 051 | 6.4 (5.8, 7.0) | 1.05 (0.98, 1.14) | 10 427 | 3.6 (3.1, 4.2) | 0.96 (0.85, 1.07) |
| 50–64 | 16 799 | 7.2 (6.6, 7.8) | 1.19 (1.11, 1.28) | 11 806 | 4.0 (3.5, 4.6) | 1.05 (0.94, 1.18) |
| ≥ 65 | 13 304 | 7.3 (6.6, 8.0) | 1.21 (1.12, 1.31) | 9 458 | 3.2 (2.7, 3.8) | 0.86 (0.73, 1.00) |
| Nationality | ||||||
| Spanish | 65 173 | 6.0 (5.7, 6.4) | 1.00 (0.98, 1.01) | 43 084 | 3.7 (3.4, 4.0) | 0.98 (0.96, 0.99) |
| Other | 3 066 | 6.6 (5.3, 8.2) | 1.10 (0.89, 1.34) | 1 364 | 6.4 (4.7, 8.6) | 1.69 (1.26, 2.26) |
| Educationf | ||||||
| Less than primary | 3 680 | 6.4 (5.4, 7.6) | 0.98 (0.83, 1.15) | 2 464 | 3.7 (2.6, 5.1) | 0.98 (0.72, 1.34) |
| Primary | 7 813 | 6.5 (5.8, 7.4) | 0.99 (0.89, 1.11) | 5 429 | 3.5 (2.9, 4.4) | 0.95 (0.79, 1.14) |
| Secondary | 14 247 | 5.8 (5.2, 6.4) | 0.88 (0.81, 0.96) | 9 724 | 3.8 (3.3, 4.4) | 1.02 (0.90, 1.14) |
| High school | 11 292 | 7.0 (6.3, 7.7) | 1.06 (0.98, 1.15) | 7 338 | 4.4 (3.8, 5.2) | 1.19 (1.05, 1.35) |
| Vocational training | 6 369 | 5.9 (5.2, 6.7) | 0.90 (0.79, 1.02) | 4 413 | 3.4 (2.8, 4.1) | 0.90 (0.75, 1.09) |
| University | 12 593 | 7.4 (6.6, 8.2) | 1.12 (1.03, 1.22) | 8 379 | 3.4 (2.9, 4.0) | 0.91 (0.79, 1.05) |
| Occupationf | ||||||
| Active worker | 28 991 | 6.9 (6.5, 7.4) | 1.07 (1.03, 1.11) | 18 186 | 4.0 (3.6, 4.5) | 1.06 (0.99, 1.13) |
| Unemployed | 4 479 | 4.4 (3.6, 5.4) | 0.68 (0.56, 0.82) | 3 623 | 4.1 (3.3, 4.9) | 1.07 (0.89, 1.30) |
| Student | 4 230 | 4.8 (4.0, 5.8) | 0.75 (0.63, 0.88) | 2 243 | 4.5 (3.5, 5.8) | 1.19 (0.94, 1.49) |
| Retired | 13 239 | 7.3 (6.6, 8.0) | 1.12 (1.04, 1.21) | 9 859 | 3.1 (2.6, 3.7) | 0.82 (0.71, 0.95) |
| Sick leave | 1 480 | 5.5 (4.0, 7.3) | 0.84 (0.63, 1.13) | 1 163 | 2.9 (2.0, 4.3) | 0.78 (0.54, 1.12) |
| Homemaker | 3 682 | 5.3 (4.4, 6.3) | 0.81 (0.68, 0.96) | 2 464 | 3.9 (3.0, 5.2) | 1.04 (0.79, 1.36) |
| Other | 1 017 | 4.7 (3.3, 6.5) | 0.72 (0.52, 1.00) | 586 | 4.4 (2.5, 7.7) | 1.16 (0.66, 2.04) |
| Occupation sectorg | ||||||
| Telecommuting | 13 159 | 7.5 (6.9, 8.1) | 1.08 (1.02, 1.13) | 1 504 | 3.8 (2.8, 5.3) | 0.96 (0.71, 1.29) |
| Retail | 1 869 | 6.3 (4.9, 8.2) | 0.91 (0.72, 1.16) | 2 462 | 3.8 (2.9, 4.9) | 0.94 (0.74, 1.19) |
| Transport | 890 | 6.5 (4.6, 9.1) | 0.93 (0.67, 1.30) | 744 | 3.6 (2.2, 5.8) | 0.90 (0.57, 1.43) |
| Security | 710 | 6.9 (4.8, 9.8) | 0.99 (0.69, 1.41) | 487 | 5.5 (3.4, 8.8) | 1.38 (0.87, 2.19) |
| Cleaning | 902 | 5.4 (3.7, 7.7) | 0.77 (0.54, 1.10) | 838 | 4.9 (3.2, 7.5) | 1.22 (0.81, 1.86) |
| Health care | 1 300 | 11.1 (9.0, 13.6) | 1.60 (1.30, 1.95) | 990 | 6.1 (4.4, 8.5) | 1.52 (1.10, 2.10) |
| Nursing home | 1 148 | 10.2 (8.0, 12.8) | 1.46 (1.17, 1.82) | 831 | 3.6 (2.2, 5.8) | 0.90 (0.56, 1.43) |
| Home caregiver | 454 | 7.3 (4.4, 11.9) | 1.04 (0.64, 1.71) | 282 | 6.0 (3.4, 10.4) | 1.50 (0.85, 2.63) |
| Other | 8 457 | 5.2 (4.6, 5.9) | 0.75 (0.68, 0.84) | 10 043 | 3.8 (3.3, 4.3) | 0.94 (0.86, 1.03) |
| Smokerf | ||||||
| No | 41 881 | 7.2 (6.7, 7.6) | 1.11 (1.08, 1.13) | 27 660 | 4.1 (3.7, 4.5) | 1.08 (1.04, 1.12) |
| Yes | 15 885 | 4.7 (4.2, 5.2) | 0.72 (0.66, 0.79) | 10 391 | 3.0 (2.5, 3.5) | 0.78 (0.68, 0.98) |
| Body mass indexf | ||||||
| < 25 | 24 935 | 6.1 (5.6, 6.6) | 0.95 (0.90, 1.00) | 15 811 | 3.6 (3.2, 4.0) | 0.94 (0.87, 1.02) |
| 25–29 | 21 786 | 6.7 (6.2, 7.2) | 1.04 (0.99, 1.09) | 14 723 | 3.8 (3.4, 4.3) | 1.01 (0.93, 1.09) |
| ≥ 30 | 11 164 | 6.8 (6.1, 7.6) | 1.05 (0.96, 1.16) | 7 589 | 4.2 (3.6, 4.9) | 1.11 (0.99, 1.25) |
| No. chronic conditionsh | ||||||
| 0 | 21 054 | 6.9 (6.4, 7.4) | 0.97 (0.93, 1.02) | 14 093 | 3.6 (3.2, 4.1) | 1.02 (0.94, 1.10) |
| 1 | 10 338 | 6.7 (6.1, 7.5) | 0.96 (0.88, 1.04) | 7 348 | 3.6 (3.0, 4.2) | 1.00 (0.88, 1.15) |
| 2 | 6 060 | 8.2 (7.3, 9.2) | 1.16 (1.04, 1.30) | 4 290 | 3.4 (2.7, 4.1) | 0.94 (0.78, 1.13) |
| ≥ 3 | 4 318 | 7.1 (6.1, 8.2) | 1.01 (0.88, 1.15) | 3 201 | 3.6 (2.7, 4.7) | 0.99 (0.77, 1.28) |
| Chronic conditionh | ||||||
| Diabetes | 4 846 | 6.7 (5.8, 7.8) | 0.96 (0.84, 1.09) | 3 366 | 3.2 (2.5, 4.1) | 0.90 (0.72, 1.14) |
| Hypertension | 12 145 | 7.6 (6.9, 8.3) | 1.07 (1.00, 1.15) | 8 565 | 3.6 (3.0, 4.2) | 1.00 (0.88, 1.13) |
| CVD | 6 034 | 7.2 (6.3, 8.1) | 1.02 (0.91, 1.14) | 4 198 | 3.2 (2.5, 4.1) | 0.90 (0.72, 1.12) |
| Cancer | 1 958 | 7.8 (6.4, 9.5) | 1.11 (0.92, 1.34) | 1 344 | 2.6 (1.8, 3.9) | 0.73 (0.50, 1.07) |
| COPD | 3 378 | 7.1 (6.0, 8.2) | 1.00 (0.86, 1.16) | 2 309 | 3.5 (2.6, 4.6) | 0.97 (0.74, 1.27) |
| Asthma | 2 907 | 7.7 (6.5, 9.0) | 1.07 (0.91, 1.26) | 2 205 | 4.0 (3.0, 5.2) | 1.11 (0.86, 1.43) |
| Sleep apnea | 2 277 | 8.0 (6.6, 9.6) | 1.12 (0.94, 1.34) | 1 790 | 4.2 (3.1, 5.6) | 1.16 (0.89, 1.53) |
| CKD | 1 352 | 8.1 (6.4, 10.2) | 1.13 (0.90, 1.42) | 1 054 | 3.5 (2.3, 5.2) | 0.97 (0.65, 1.44) |
| IST | 1 208 | 7.5 (5.9, 9.6) | 1.05 (0.83, 1.33) | 952 | 3.9 (2.6, 6.0) | 1.10 (0.74, 1.64) |
| CLD | 510 | 6.2 (4.0, 9.6) | 0.86 (0.56, 1.31) | 475 | 2.8 (1.5, 5.4) | 0.80 (0.42, 1.49) |
| ACTD | 1 118 | 10.0 (7.8, 12.7) | 1.37 (1.08, 1.73) | 1 031 | 3.7 (2.2, 5.9) | 1.02 (0.63, 1.65) |
| Disability, % | ||||||
| No | 62 631 | 6.1 (5.8, 6.5) | 1.00 (0.99, 1.01) | 41 718 | 3.7 (3.4, 4.1) | 1.00 (0.99, 1.02) |
| < 33 | 583 | 5.4 (3.5, 8.1) | 0.87 (0.57, 1.33) | 416 | 3.0 (1.7, 5.2) | 0.79 (0.45, 1.40) |
| 33–65 | 1 779 | 6.3 (5.0, 7.9) | 1.03 (0.82, 1.28) | 1 250 | 3.8 (2.6, 5.5) | 1.01 (0.70, 1.46) |
| ≥ 66 | 946 | 5.9 (4.2, 8.2) | 0.96 (0.69, 1.33) | 650 | 3.9 (2.1, 7.3) | 1.05 (0.57, 1.92) |
| Household size, no. residents | ||||||
| 1 | 5 542 | 6.4 (5.6, 7.3) | 1.06 (0.94, 1.19) | 2 838 | 2.6 (2.0, 3.3) | 0.68 (0.52, 0.88) |
| 2 | 15 794 | 6.9 (6.3, 7.6) | 1.15 (1.06, 1.24) | 11 295 | 3.3 (2.8, 3.8) | 0.87 (0.75, 1.00) |
| 3–5 | 43 180 | 5.8 (5.4, 6.2) | 0.95 (0.92, 0.99) | 28 021 | 4.1 (3.7, 4.6) | 1.09 (1.04, 1.15) |
| ≥ 6 | 3 771 | 4.9 (3.8, 6.3) | 0.82 (0.64, 1.04) | 2 297 | 3.3 (2.3, 4.9) | 0.88 (0.60, 1.28) |
| Census tract income, percentile | ||||||
| < 5th | 3 267 | 5.8 (4.1, 8.2) | 0.96 (0.69, 1.36) | 1 957 | 5.4 (3.9, 7.4) | 1.43 (1.05, 1.95) |
| 5th–24th | 14 915 | 6.1 (5.3, 7.0) | 1.01 (0.88, 1.15) | 9 699 | 4.0 (3.2, 4.9) | 1.05 (0.88, 1.27) |
| 25th–49th | 17 261 | 5.9 (5.2, 6.8) | 0.98 (0.87, 1.12) | 11 173 | 3.7 (3.2, 4.4) | 0.99 (0.85, 1.15) |
| 50th–74th | 15 642 | 5.7 (5.0, 6.6) | 0.95 (0.84, 1.08) | 10 280 | 3.9 (3.3, 4.7) | 1.03 (0.89, 1.20) |
| 75–94th | 13 587 | 6.2 (5.4, 7.1) | 1.02 (0.90, 1.17) | 9 146 | 3.5 (2.8, 4.2) | 0.92 (0.77, 1.11) |
| ≥ 95th | 3 615 | 7.4 (5.8, 9.3) | 1.22 (0.96, 1.54) | 2 196 | 2.4 (1.6, 3.6) | 0.63 (0.42, 0.96) |
| Municipality size, no. inhabitants | ||||||
| < 5000 | 12 167 | 5.3 (4.6, 6.1) | 0.87 (0.76, 1.00) | 8 373 | 4.6 (3.7, 5.8) | 1.23 (0.99, 1.52) |
| 5000–19 999 | 14 439 | 4.7 (4.1, 5.3) | 0.77 (0.68, 0.87) | 9 565 | 3.4 (2.7, 4.3) | 0.90 (0.73, 1.11) |
| 20 000–99 999 | 20 652 | 4.9 (4.4, 5.4) | 0.81 (0.74, 0.89) | 13 189 | 3.4 (2.8, 4.0) | 0.90 (0.77, 1.04) |
| ≥ 100 000 | 21 029 | 7.7 (7.1, 8.4) | 1.28 (1.21, 1.35) | 13 324 | 4.0 (3.5, 4.6) | 1.05 (0.95, 1.17) |
Note. ACTD = autoimmune connective tissue disease; CI = confidence interval; CKD = chronic kidney disease; CLD = chronic liver disease; COPD = chronic obstructive pulmonary disease; CVD = cardiovascular disease; ENE–COVID = Spanish National Seroepidemiological Survey of SARS-CoV-2 Infection/Estudio Nacional de Sero-Epidemiología de la Infección por SARS-CoV-2 en España; IST = immunosuppressive therapy; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Participants who received the point-of-care test in at least 1 of the 3 rounds of the first survey phase.
Proportion of participants who had detectable immunoglobulin G antibodies against SARS-CoV-2 in any round of the first survey phase by the point-of-care test.
Seropositivity ratio with respect to all combined categories of a given characteristic.
Participants who tested negative by all received point-of-care tests during the first phase and underwent the test in the single round of the second survey phase.
Proportion of seronegative participants in the first phase who had detectable immunoglobulin G antibodies against SARS-CoV-2 in the single round of the second survey phase by the point-of-care test.
Among participants aged 17 y or older. Body mass index is defined as weight in kilograms divided by the square of height in meters.
Among active workers.
Among participants aged 40 y or older. Number of chronic conditions computed from those listed in the table.
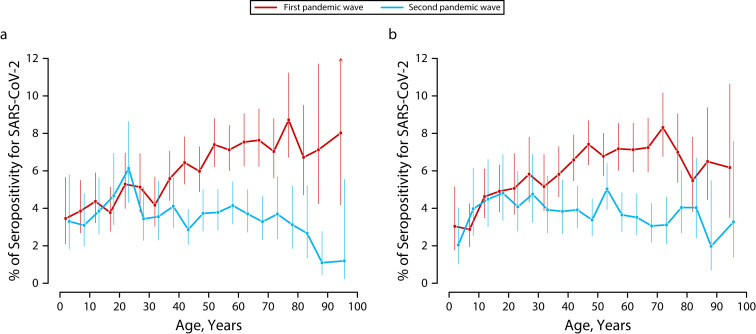
Seropositivity increased with age, being lower among those younger than 20 years (4%) and higher in those 50 years and older (7%). This pattern was observed in both males and females (Figure 2), although seroprevalence peaked at approximately 75 years in women only. As seen in Table 1, prevalence of infection was higher in people with a bachelor’s degree or higher (SPR = 1.12; 95% CI = 1.03, 1.22), particularly among men (Table A), those living in census tracts with higher income (SPR = 1.22; 95% CI = 0.96, 1.54), and those living in cities (SPR = 1.28; 95% CI = 1.21, 1.35). Health care and nursing home workers were more affected than were workers in other occupational sectors with activity during the lockdown (SPR = 1.60; 95% CI = 1.30, 1.95 and SPR = 1.46; 95% CI = 1.17, 1.82, respectively). Smokers had lower seroprevalence than did nonsmokers (4.7% vs 7.2%).
FIGURE 2—
New Seropositive Participants for SARS-CoV-2 as Determined by the Point-of-Care Test in the First and Second Pandemic Waves by Age Among (a) Men and (b) Women: ENE–COVID, Spain, April 27–June 22 and November 16–30, 2020
Note. ENE–COVID = Spanish National Seroepidemiological Survey of SARS-CoV-2 Infection/Estudio Nacional de Sero-Epidemiología de la Infección por SARS-CoV-2 en España; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Vertical lines represent 95% confidence intervals.
During the second wave, 3.8% of participants were newly seropositive. The geographical distribution was more diffuse (Figure 1). Highest de novo seroprevalences were observed in northeast Spain, whereas the middle of the country was still more affected. Again, no differences were observed by gender, but the age distribution was different in men and women (Figure 2). The highest seroprevalence was observed in those aged 20 to 29 years and in men, with a clear downward trend after aged 75 years. This trend was not as clear in females, who had a constant percentage of new seropositive participants from children aged 5 to 9 years to adults aged 80 to 84 years. In this pandemic wave, Spanish participants were less affected than were those from other countries (3.7 vs 6.4%; Table 1), with no differences between men and women (Table A).
Those who worked in the security (SPR = 1.38; 95% CI = 0.87, 2.19), cleaning (SPR = 1.22; 95% CI = 0.81, 1.86), health care (SPR = 1.52; 95% CI = 1.10, 2.10), and home care (SPR = 1.50; 95% CI = 0.85, 2.63) sectors had higher seropositivity, whereas nursing home workers were not among those more affected (SPR = 0.90; 95% CI = 0.56, 1.43). The analysis by gender showed high risk in security workers among men, cleaners and nursing home workers among women, and health care workers among both genders (Table A). Unlike what was observed in the first wave, the most affected areas were low-income census tracts (SPR = 1.43; 95% CI = 1.05, 1.95) and smaller towns (SPR = 1.23; 95% CI = 0.99, 1.52). Finally, de novo seropositivity was also lower among smokers than those who did not smoke (3.0% vs 4.1%).
Table 2 presents the same figures according to COVID-19–related factors. As expected, in both waves symptoms and antecedents of a positive PCR result were strongly associated with a positive serological result (SPR > 2). Every type of contact with those with confirmed or suspected COVID-19 increased the risk, but estimates were highest if the contact was with household members, especially in the second pandemic wave (SPR = 3.65 and 9.25 during the first and second waves, respectively, for cohabitant with confirmed COVID-19; SPR = 2.46 and 6.97 for cohabitant suspected to have COVID-19 in the corresponding wave). Analyses by gender did not show remarkable differences in the influence of these factors (Appendix Table B).
TABLE 2—
New Seropositive Participants for SARS-CoV-2 as Determined by the Point-of-Care Test in the First and Second Pandemic Waves by COVID-19–Related Characteristics: ENE–COVID, Spain, April 27–June 22 and November 16–30, 2020
| Characteristica | First Pandemic Wave | Second Pandemic Wave | ||||
| No. of Participantsb | Seropositivity,c % (95% CI) | Seropositivity Ratiod (95% CI) | No. of Participantse | Seropositivity,f % (95% CI) | Seropositivity Ratiod (95% CI) | |
| Symptoms compatible with COVID-19g | ||||||
| Asymptomatic | 42 275 | 3.7 (3.4, 3.9) | 0.61 (0.57, 0.64) | 35 674 | 1.8 (1.6, 2.1) | 0.48 (0.44, 0.53) |
| Paucisymptomatic | 15 663 | 5.1 (4.6, 5.6) | 0.84 (0.77, 0.91) | 5 715 | 5.1 (4.3, 6.0) | 1.34 (1.17, 1.53) |
| Symptomatic | 10 346 | 16.5 (15.3, 17.8) | 2.73 (2.59, 2.87) | 3 062 | 22.3 (20.0, 24.7) | 5.90 (5.45, 6.38) |
| Time since symptom onset, d | ||||||
| ≤ 14 | 362 | 15.2 (10.9, 20.8) | 2.51 (1.83, 3.46) | 420 | 8.3 (5.5, 12.5) | 2.21 (1.46, 3.33) |
| > 14h | 9 984 | 16.5 (15.3, 17.8) | 2.73 (2.60, 2.88) | 2 642 | 24.6 (22.1, 27.4) | 6.53 (6.01, 7.08) |
| PCR status | ||||||
| Never tested | 62 198 | 5.9 (5.5, 6.2) | 0.97 (0.96, 0.99) | 34 365 | 1.8 (1.6, 2.0) | 0.47 (0.42, 0.51) |
| Negative | 5 538 | 3.4 (2.8, 4.1) | 0.56 (0.47, 0.67) | 8 749 | 3.0 (2.5, 3.6) | 0.80 (0.68, 0.94) |
| Positive ≤ 14 d | 33 | 58.5 (34.0, 79.4) | 9.68 (6.38, 14.7) | 100 | 47.1 (34.7, 59.9) | 12.47 (9.49, 16.4) |
| Positive > 14 dh | 353 | 66.4 (58.6, 73.4) | 10.99 (9.82, 12.3) | 1 192 | 64.1 (59.9, 68.0) | 16.96 (15.6, 18.4) |
| Awaiting result | 161 | 9.3 (4.6, 17.8) | 1.53 (0.78, 3.03) | 44 | 0.9 (0.2, 4.4) | 0.25 (0.05, 1.19) |
| Contact with confirmed case | ||||||
| No contact | 60 591 | 5.0 (4.7, 5.3) | 0.83 (0.80, 0.85) | 36 519 | 1.9 (1.7, 2.2) | 0.51 (0.47, 0.56) |
| Household member | 1 897 | 22.1 (18.9, 25.6) | 3.65 (3.15, 4.24) | 1 424 | 35.0 (30.8, 39.4) | 9.25 (8.30, 10.3) |
| Noncohabitating family/friend | 2 518 | 10.6 (9.1, 12.4) | 1.76 (1.52, 2.03) | 3 145 | 11.1 (9.3, 13.2) | 2.94 (2.55, 3.38) |
| Co-worker | 2 169 | 10.9 (9.1, 13.0) | 1.80 (1.52, 2.13) | 1 916 | 5.3 (4.2, 6.7) | 1.41 (1.14, 1.75) |
| Cleaning person/caregiver | 127 | 11.7 (6.4, 20.6) | 1.94 (1.07, 3.52) | 102 | 11.6 (4.9, 25.1) | 3.07 (1.35, 7.02) |
| Patient/client | 1 437 | 10.8 (8.8, 13.1) | 1.78 (1.47, 2.16) | 755 | 6.5 (4.5, 9.3) | 1.72 (1.19, 2.48) |
| Classmate | N/A | N/A | N/A | 1 033 | 4.8 (3.3, 6.9) | 1.27 (0.90, 1.80) |
| Contact with symptomatic person | ||||||
| No contact | 55 507 | 4.3 (4.0, 4.6) | 0.71 (0.68, 0.74) | 40 182 | 2.8 (2.5, 3.1) | 0.74 (0.70, 0.78) |
| Household member | 5 465 | 14.9 (13.3, 16.6) | 2.46 (2.24, 2.70) | 1 083 | 26.3 (22.3, 30.8) | 6.97 (5.96, 8.15) |
| Noncohabitating family/friend | 3 215 | 11.7 (10.1, 13.5) | 1.93 (1.70, 2.20) | 1 377 | 12.4 (9.9, 15.5) | 3.29 (2.69, 4.04) |
| Co-worker | 2 991 | 11.0 (9.5, 12.7) | 1.82 (1.59, 2.08) | 945 | 6.7 (4.9, 8.9) | 1.76 (1.33, 2.34) |
| Cleaning person/caregiver | 167 | 6.6 (3.4, 12.2) | 1.09 (0.57, 2.07) | 36 | 5.4 (1.7, 16.2) | 1.44 (0.46, 4.55) |
| Patient/client | 1 499 | 10.1 (8.2, 12.4) | 1.67 (1.37, 2.05) | 544 | 8.2 (5.4, 12.4) | 2.18 (1.44, 3.29) |
| Classmate | N/A | N/A | N/A | 470 | 4.7 (2.9, 7.8) | 1.26 (0.76, 2.07) |
Note. CI = confidence interval; ENE–COVID = Spanish National Seroepidemiological Survey of SARS-CoV-2 Infection/Estudio Nacional de Sero-Epidemiología de la Infección por SARS-CoV-2 en España; N/A = not available; PCR = polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
For the first pandemic wave, information up to the last round of participation of the first survey phase or the first round with positive result as determined by the point-of-care test; for the second pandemic wave, information after the end of the first wave (July 1, 2020).
Participants who received the point-of-care test in at least 1 of the 3 rounds of the first survey phase.
Proportion of participants who had detectable immunoglobulin G antibodies against SARS-CoV-2 in any round of the first survey phase as determined by the point-of-care test.
Seropositivity ratio with respect to all combined categories of a given characteristic.
Participants who tested negative by all received point-of-care tests during the first phase and underwent the test in the single round of the second survey phase.
Proportion of seronegative participants in the first phase who had detectable immunoglobulin G antibodies against SARS-CoV-2 in the single round of the second survey phase as determined by the point-of-care test.
Asymptomatic (no symptoms), paucisymptomatic (1–2 symptoms without anosmia/ageusia), and symptomatic (anosmia/ageusia or at least 3 symptoms among fever, chills, severe tiredness, sore throat, cough, shortness of breath, headache, and nausea/vomiting/diarrhea).
For the first pandemic wave > 14 d before any round of participation of the first survey phase or before the first round with positive result as determined by the point-of-care test.
Seropositivity determined by the CMIA decreased to 4.9% and 3.3% during the first and second pandemic waves, respectively. Results were like those for the point-of-care test, although estimates were less precise because the CMIA was offered to only a random subcohort in the second wave (Appendix Tables C and D). In sensitivity analyses combining both tests, seropositivity ranged from 3.9% (95% CI = 3.6%, 4.2% when both tests were positive) to 7.3% (95% CI = 6.9%, 7.7% when either test was positive) in the first wave and from 2.6% (95% CI = 1.9%, 3.4%) to 4.3% (95% CI = 3.5%, 5.3%) in the second wave. Second to first wave seropositivity ratios were 0.65 (95% CI = 0.48, 0.89) for both tests positive and 0.58 (95% CI = 0.47, 0.72) for either test positive, which were like ratios obtained for each test separately: 0.63 (95% CI = 0.56, 0.70) for the point-of-care test and 0.68 (95% CI = 0.52, 0.89) for the CMIA.
DISCUSSION
According to ENE–COVID, between 5% and 6% of the noninstitutionalized population had been infected by SARS-CoV-2 by June 2020 in Spain, during the first pandemic wave, which suggests 2.3 to 2.8 million people were infected. To these figures, we should add those occurring in nursing homes, not included in the ENE–COVID design, which were heavily affected.5 Our results suggest that, at that moment, fewer than 1 in 10 infections were diagnosed and registered in the RENAVE (the National Epidemiological Surveillance System).6 There was a severe shortage of PCR tests, which were reserved for hospitalized patients and for symptomatic essential workers, mainly health care professionals—a sector with a clear predominance of women (74%).7 This created a distorted picture of the situation, with more than 40% of notified infected individuals among people 70 years and older and a predominance of women over men.1
By contrast, the first 3 rounds of ENE–COVID showed similar seroprevalences in males and females, whereas the differences by age were much attenuated. Regarding the geographical distribution, ENE–COVID confirmed the higher prevalence in Madrid, Castilla-La Mancha, and Castilla y León (the center of the country) and Barcelona (in the northeast). Navarre and La Rioja were hotspots according to the RENAVE data,1 but they did not have very high seroprevalence figures. This discordance suggests differences in the availability of diagnostic tests across Spanish autonomous communities.
The first pandemic wave in Spain was dominated by 2 early clades closely related to Asian SARS-CoV-2 variants.8,9 The outbreak overwhelmed the health care system in several Spanish regions.10 Health care workers were overstressed and insufficiently protected11,12 and, according to our results, 1 in 10 was infected during this period. The national lockdown, enforced at the peak of the pandemic, included school closure, workplace closure, and mobility restrictions and served to reverse the trend and contain the viral transmission.9,13 At the end of June 2020, the country slowly recovered mobility and economic activity, although some restrictions were maintained, including the mandatory use of face masks. A new variant, B.1.177 (initially named 20E.EU1), with increased transmissibility was identified early that summer in the country and later became dominant in Europe.14
The second pandemic wave started to escalate in July to August 2020, and the Spanish government declared a second state of emergency at the peak of the second wave, October 25, but did not impose a national lockdown. In this case, autonomous communities implemented different public health interventions over time. By the middle of November, when the last round of ENE–COVID started, there were more than 1 million new cases reported to the RENAVE, many of them asymptomatic.6 However, according to ENE–COVID estimates, between 3.3% and 3.8% of house-dwelling people were newly infected by SARS-CoV-2, that is, approximately 1.5 to 1.6 million people. The highest de novo seropositivity was observed in the northeast of the country.
Again, men and women were equally affected, but the age distribution was different during the second wave. Among previously uninfected men, the highest seroprevalence occurred at age 20 to 29 years, and the oldest age groups had the lowest risk. In women, the distribution was somehow more homogeneous, but lowest rates were also observed among older women. These disparities correlate well with the reported differences in risk perception toward COVID-19 by age and gender in Spain,15 suggesting that older people, particularly men, stayed at home more often and tended to avoid social contact,4 reducing their infection risk.16 In women, differences were less obvious, and although they have been reported to be more cautious,15 their role at home and as caregivers may have presented more opportunities to become infected.17
Big cities, with greater international exchange and greater population density, had a higher seroprevalence during the first wave. However, the pandemic moved from the cities to the rural areas during the summer, with smaller municipalities having the highest seroprevalence in the second wave. Restrictions on traveling outside Spain, together with increased concern about becoming infected abroad, made people spend their summer holidays inside the country, choosing less populated places.
The ENE–COVID study was not designed to explore socioeconomic differences in detail, but some individual and ecological information allowed us to explore this aspect. Highly educated men and people living in wealthier census tracts were more frequently infected at the beginning of the pandemic, possibly because of their greater international mobility.9 The first pandemic wave took everybody by surprise, and even though some reports found stronger effects in areas with lower socioeconomic status within big cities, such as Barcelona,18 the national lockdown seemed to attenuate them. Later, as the virus was widely disseminated, disadvantaged socioeconomic groups were at higher risk of infection. During the second pandemic wave, participants living in lower income census tracts and non-Spanish people were more affected. These socioeconomic differences have been observed in other countries,19–23 and a combination of factors, related to work and home conditions, may explain them.24,25 In fact, the first big outbreaks that summer occurred in occupational settings,26 and, as ENE–COVID shows, essential low-wage occupations, such as cleaners and home caregivers, were at higher risk for infection.
Living with someone who was confirmed or suspected to be infected was the strongest risk factor for being seropositive, especially in the second wave, reflecting the difficulty of isolating those infected with SARS-CoV-2 at home. Even though some autonomous communities provided specific places to isolate infected people if needed (e.g., hotels), these resources were not generally available. Also, in the second wave, risk of infection was higher after contact with friends or noncohabiting relatives who were confirmed or suspected to be infected than after contact at work, suggesting lower success of public health measures in informal settings.
Of note, in both waves, smokers had lower seroprevalence values. It is unlikely that this result is attributable to selection bias, and our data support studies that suggest smokers have a reduced risk of infection.27
Strengths and Limitations
This study updates the epidemiological information on the first pandemic wave in Spain. It considered 3 consecutive survey rounds and describes for the first time, to our knowledge, the characteristics of the second pandemic wave. With a population-based design and high percentages of participation, ENE–COVID provided an accurate representation of the situation of SARS-CoV-2 infection in the whole country. In this sense, our random selection sampling design, as well as our use of poststratification sampling weights, which served to correct regional income, age, and gender differences in participation, are 2 of the strongest points of ENE–COVID. The National Centre for Microbiology of the Instituto de Salud Carlos III selected the serological tests after carefully screening those available.
ENE–COVID also has several limitations. It did not study the situation of institutionalized people, as they required a different approach. Although only 6% of participants older than 75 years reside in nursing homes,28 they suffered many outbreaks with very high mortality.4,5 Also, the information relies on detection of anti-SARS-CoV-2 IgG antibodies, which appear 1 to 3 weeks after infection.29 Apart from the false positive and negative results associated with the characteristics of the test, a low proportion of infected people, particularly those who are asymptomatic, do not seroconvert,29,30 whereas those who become positive may test negative after several months.29 Seroreversion, however, is not an issue here, because ENE–COVID started at the end of April, just a month after the peak of the first pandemic wave.
Finally, the second pandemic wave was still trending downward when the study took place, leading us to underestimate new seroprevalence figures. Also, this last round of the survey had lower participation, possibly because of a loss of interest in being tested given the greater availability of diagnostic and serological tools. Still, almost 7 of 10 participants who were previously seronegative agreed to participate. Results for this wave are based mainly on the point-of-care test, because venipuncture was offered only to a subsample of seronegative participants to facilitate the fieldwork in primary health centers, which were still severely overloaded because of the pandemic.31
Conclusions
ENE–COVID served to characterize the first 2 pandemic waves of SARS-CoV-2 and provided useful information when data on COVID-19 cases and new infections were insufficient. People living in the center of Spain, those living in cities, and those with higher education were more affected during the first wave. However, the virus spread across the country, particularly toward the northeast, and more disadvantaged groups were at higher risk for infection after lifting the national lockdown. Health workers and individuals in other occupations who take care of vulnerable people suffered higher rates of infection. In both waves, living with someone who had COVID-19 strongly influenced the probability of being infected.
Public Health Implications
From a public health surveillance standpoint, ENE–COVID has provided health authorities key and timely information on the COVID-19 pandemic as follows:
-
•
Seroprevalence studies are useful for providing information when there is incomplete case ascertainment and there may be a substantial proportion of asymptomatic infections.
-
•
Contrary to the picture drawn by the National Epidemiological Surveillance System, we learned that the virus infected men and women equally and that all age groups were at risk.
-
•
According to seroprevalence figures, the proportion of people infected during these 2 pandemic waves was insufficient to guarantee herd immunity.
-
•
Health care workers suffered the brunt of the new pandemic, with seroprevalence figures that were almost double those found in the overall population.
-
•
Living with an infected person was the strongest avoidable risk factor; public health strategies should give households more attention.
-
•
Older people, who were at higher risk of death, had a lower relative infection rate in the second wave.
-
•
Socioeconomic differences appeared during the second wave. In situations like this, specific measures are needed to protect disadvantaged groups.
It is important to highlight the added value of the longitudinal design of ENE–COVID, which allowed us to explore changes in the epidemiological characteristics of the pandemic over time.
ACKNOWLEDGMENTS
This work was supported by the Spanish Ministry of Health, the Institute of Health Carlos III (Ministry of Science and Innovation), and the National Health System, including the Health Services of all autonomous communities and autonomous cities: Servicio Andaluz de Salud, Servicio Aragonés de Salud, Servicio de Salud del Principado de Asturias, Servei de Salut Illes Balears, Servicio Canario de la Salud, Servicio Cántabro de Salud, Servicio de Salud de Castilla-La Mancha, Servicio de Salud de Castilla y León, Servei Català de Salut, Conselleria de Sanitat Universal i Salut Pública de la Generalitat Valenciana, Servicio Extremeño de Salud, Servizo Galego de Saúde, Servicio Riojano de Salud, Servicio Madrileño de Salud, Servicio Murciano de Salud, Servicio Navarro de Salud-Osasunbidea and Instituto de Salud Pública y Laboral de Navarra, Servicio Vasco de Salud-Osakidetza, and Instituto de Gestión Sanitaria.
We thank all the nurses, general practitioners, administrative personnel, and other health care workers who collaborated in this study and all participants. This study is the result of the efforts of many professionals and the trust and generosity of more than 68 000 participants who understood the importance of providing time, information, and samples to learn about the COVID-19 pandemic in Spain. The Spanish Institute of Statistics provided the random selection of households and the information required to contact participants.
Collaborators of the ENE–COVID Study Group (Spanish National Seroepidemiological Survey of SARS-CoV-2 Infection/Estudio Nacional de Sero-Epidemiología de la Infección por SARS-CoV-2 en España) are listed in the Appendix (available as a supplement to the online version of this article at http://www.ajph.org).
CONFLICTS OF INTEREST
M. A. Hernán is a consultant for Cytel and adviser for ProPublica. The other authors have no conflicts of interest to declare.
HUMAN PARTICIPANT PROTECTION
The Institute of Health Carlos III institutional review board (ISCIII Committee for Ethical Research) approved the study (register No. PI 39_2020).
REFERENCES
- 1.Working Group for the Surveillance and Control of COVID-19 in Spain. The first wave of the COVID-19 pandemic in Spain: characterisation of cases and risk factors for severe outcomes, as at 27 April 2020. Euro Surveill. 2020;25(50):2001431. doi: 10.2807/1560-7917.ES.2020.25.50.2001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Instituto de Salud Carlos III. National Study of SARS-CoV-2 Sero-Epidemiology in Spain (ENE–COVID) 2020. https://portalcne.isciii.es/enecovid19
- 4.Pastor-Barriuso R, Pérez-Gómez B, Hernán MA, et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: nationwide seroepidemiological study. BMJ. 2020;371:m4509. doi: 10.1136/bmj.m4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IMSERSO. Informe Del Grupo de Trabajo COVID 19 y Residencias. 2020. https://www.imserso.es/InterPresent1/groups/imserso/documents/binario/gtcovid_residencias_vf.pdf
- 6.Instituto de Salud Carlos III. COVID-19 in Spain. 2020. https://cnecovid.isciii.es/covid19
- 7.Nova IP. Radiografía del papel de la mujer en la sanidad española. Redacción Médica. 2018https://www.redaccionmedica.com/secciones/sanidad-hoy/radiografia-del-papel-de-la-mujer-en-la-sanidad-espanola-1050
- 8.Díez-Fuertes F, Iglesias-Caballero M, García-Pérez J, et al. A founder effect led early SARS-CoV-2 transmission in Spain. J Virol. 2021;95(3):e01583–e015820. doi: 10.1128/JVI.01583-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López MG, Chiner-Oms Á, García de Viedma D, et al. The first wave of the COVID-19 epidemic in Spain was associated with early introductions and fast spread of a dominating genetic variant. Nat Genet. 2021;53(10):1405–1414. doi: 10.1038/s41588-021-00936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Alés G, Domingo-Relloso A, Arribas JR, Quintana-Díaz M, Hernán MA COVID@HULP Group. Critical care requirements under uncontrolled transmission of SARS-CoV-2. Am J Public Health. 2021;111(5):923–926. doi: 10.2105/AJPH.2020.306151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso J, Vilagut G, Mortier P, et al. Mental health impact of the first wave of COVID-19 pandemic on Spanish healthcare workers: a large cross-sectional survey. Rev Psiquiatr Salud Ment (Engl Ed). 2021;14(2):90–105. doi: 10.1016/j.rpsm.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Gil MT, González-Blázquez C, Parro-Moreno AI, et al. Nurses’ perceptions and demands regarding COVID-19 care delivery in critical care units and hospital emergency services. Intensive Crit Care Nurs. 2021;62:102966. doi: 10.1016/j.iccn.2020.102966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam N, Sharp SJ, Chowell G, et al. Physical distancing interventions and incidence of coronavirus disease 2019: natural experiment in 149 countries. BMJ. 2020;370:m2743. doi: 10.1136/bmj.m2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodcroft EB, Zuber M, Nadeau S, et al. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature. 2021;595(7869):707–712. doi: 10.1038/s41586-021-03677-y. [DOI] [PubMed] [Google Scholar]
- 15.Recio-Vivas AM, Font-Jiménez I, Mansilla-Domínguez JM, et al. Fear and attitude towards SARS-CoV-2 (COVID-19) infection in Spanish population during the period of confinement. Int J Environ Res Public Health. 2022;19(2):834. doi: 10.3390/ijerph19020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrasfay T, Wu Q, Lee H, Crimmins EM. Adherence to social-distancing and personal hygiene behavior guidelines and risk of COVID-19 diagnosis: evidence from the Understanding America Study. Am J Public Health. 2022;112(1):169–178. doi: 10.2105/AJPH.2021.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayittey FK, Dhar BK, Anani G, Chiwero NB. Gendered burdens and impacts of SARS-CoV-2: a review. Health Care Women Int. 2020;41(11–12):1210–1225. doi: 10.1080/07399332.2020.1809664. [DOI] [PubMed] [Google Scholar]
- 18.Baena-Díez JM, Barroso M, Cordeiro-Coelho SI, Díaz JL, Grau M. Impact of COVID-19 outbreak by income: hitting hardest the most deprived. J Public Health (Oxf). 2020;42(4):698–703. doi: 10.1093/pubmed/fdaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plümper T, Neumayer E. The pandemic predominantly hits poor neighbourhoods? SARS-CoV-2 infections and COVID-19 fatalities in German districts. Eur J Public Health. 2020;30(6):1176–1180. doi: 10.1093/eurpub/ckaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrat F, de Lamballerie X, Rahib D, et al. Antibody status and cumulative incidence of SARS-CoV-2 infection among adults in three regions of France following the first lockdown and associated risk factors: a multicohort study Int J Epidemiol. 20215051458–1472.. [Erratum in: Int J Epidemiol. Aug 28, 2021]. 10.1093/ije/dyab110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saarinen S, Moustgaard H, Remes H, Sallinen R, Martikainen P. Income differences in COVID-19 incidence and severity in Finland among people with foreign and native background: a population-based cohort study of individuals nested within households. PLoS Med. 2022;19(8):e1004038. doi: 10.1371/journal.pmed.1004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):703–706. doi: 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanijahani A, Iezadi S, Gholipour K, Azami-Aghdash S, Naghibi D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health. 2021;20(1):248. doi: 10.1186/s12939-021-01582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burström B, Tao W. Social determinants of health and inequalities in COVID-19. Eur J Public Health. 2020;30(4):617–618. doi: 10.1093/eurpub/ckaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson G, Frank JW, Naylor CD, Wodchis W, Feng P. Using socioeconomics to counter health disparities arising from the COVID-19 pandemic. BMJ. 2020;369:m2149. doi: 10.1136/bmj.m2149. [DOI] [PubMed] [Google Scholar]
- 26.National COVID-19 Outbreak Monitoring Group. COVID-19 outbreaks in a transmission control scenario: challenges posed by social and leisure activities, and for workers in vulnerable conditions, Spain, early summer 2020. Euro Surveill. 2020;25(35):2001545. doi: 10.2807/1560-7917.ES.2020.25.35.2001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paleiron N, Mayet A, Marbac V, et al. Impact of tobacco smoking on the risk of COVID-19: a large scale retrospective cohort study. Nicotine Tob Res. 2021;23(8):1398–1404. doi: 10.1093/ntr/ntab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abellan García A, Aceituno Nieto P, Fernández Morales I, Ramiro Fariñas D, Pujol Rodríguez R.Una estimación de la población que vive en residencias de mayores—EnR. 2020. http://envejecimientoenred.es/una-estimacion-de-la-poblacion-que-vive-en-residencias-de-mayores
- 29.Wei J, Matthews PC, Stoesser N, et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat Commun. 2021;12(1):6250. doi: 10.1038/s41467-021-26479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen LR, Sami S, Vuong N, et al. Lack of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a large cohort of previously infected persons. Clin Infect Dis. 2021;73(9):e3066–e3073. doi: 10.1093/cid/ciaa1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Aguilar C, Casado-Aranda LA, Farrés Fernández M, Minué Lorenzo S. Has COVID-19 changed the workload for primary care physicians? The case of Spain. Fam Pract. 2021;38(6):780–785. doi: 10.1093/fampra/cmab028. [DOI] [PMC free article] [PubMed] [Google Scholar]