Table 4.
Estimate of the Average Causal Effect for the Palivizumab Case Study Obtained Using Strategies 2 and 3, Using an Outcome Model Controlled for Confounders and Propensity Score
| Sensitivity Parameters | Trial Size |
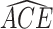 a
a
|
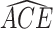 , %
, %
|
95% CI | Mean Gestational Age, Treated | Mean Gestational Age, Untreated |
|---|---|---|---|---|---|---|
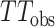
| ||||||
| N/A | 1,560 | −0.003 | −0.3% | −0.05, 0.05 | 26.5 | 27.2 |
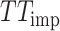
| ||||||
| (0,0) | 2,643 | −0.002 | −0.2% | −0.04, 0.04 | 26.9 | 27.7 |
| (−4,0) | 3,659 | −0.010 | −1.0% | −0.04, 0.03 | 26.9 | 25.5 |
| (0,−4) | 2,985 | 0.013 | 1.3% | −0.03, 0.05 | 24.2 | 27.7 |
| (−4,-4) | 3,964 | 0.006 | 0.6% | −0.02, 0.04 | 24.2 | 25.5 |
Abbreviations: ACE, average causal effect; CI, confidence interval; N/A, not applicable; 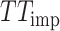 , target trial emulated from observed and imputed data;
, target trial emulated from observed and imputed data; 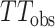 , target trial emulated from observed data.
, target trial emulated from observed data.
a The ACE is expressed as a risk difference both in absolute value and in percentage risk difference The sensitivity parameters are listed in order 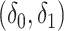 . With sensitivity parameter
. With sensitivity parameter 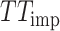 (0,0), the data are assumed missing at random. In all other cases for
(0,0), the data are assumed missing at random. In all other cases for 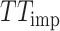 it is assumed missing not at random. This is not applicable for
it is assumed missing not at random. This is not applicable for 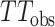 , which has complete data.
, which has complete data.
