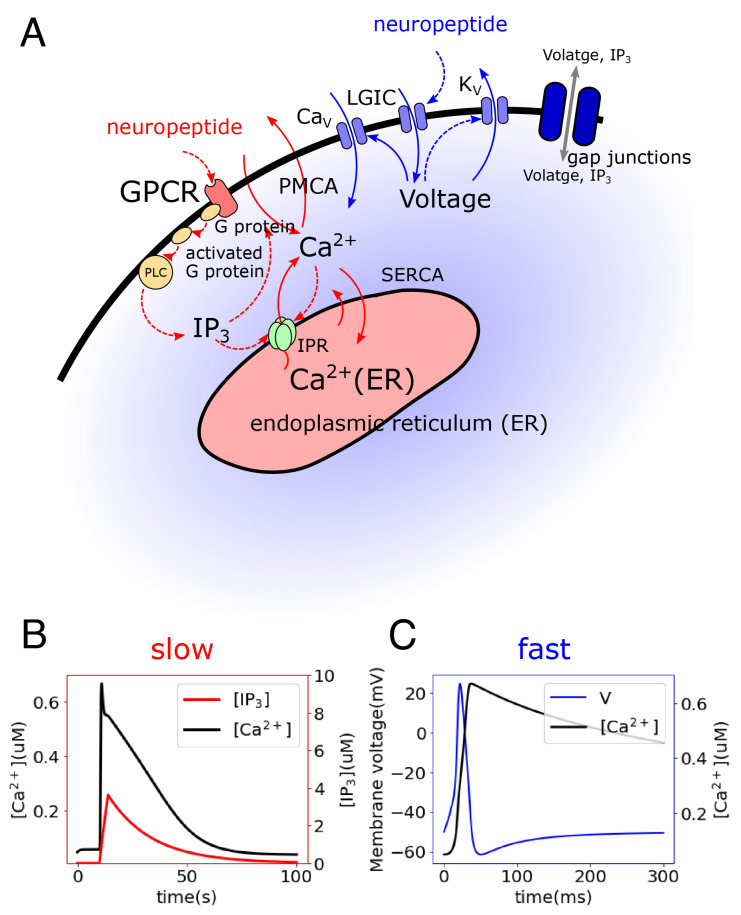
Fig. 3.
Modeled single-cell dynamics. (A) Intracellular calcium signaling model including two pathways: (i) In the slow pathway (red), neuropeptides bind a G protein-coupled receptor (GPCR) and activate a G protein, which activates phospholipase C (PLC) and hydrolyzes phosphatidylinositol bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3), which plays a role of the second messenger for calcium signaling. IP3 can bind to IP3 receptors (IPR) on the endoplasmic reticulum (ER) membrane and thus cause the release of Ca2+ from the ER. Ca2+ is extruded to the extracellular space through plasma membrane Ca2+ ATPase (PMCA) and is recycled to the ER through sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) (30, 91). (ii) The fast pathway (blue) is initiated by the binding of neuropeptides on ionotropic receptors, which activate ligand-gated ion channels (LGIC) and depolarize the cellular membrane (92). The elevated membrane potential activates calcium channels (L-type/T-type), triggering a large influx of Ca2+ from the extracellular space and invoking further depolarization. Meanwhile, the high membrane potential inactivates calcium channels and activates potassium channels, resulting in membrane repolarization (71). (B) Simulated slow dynamics showing change of Ca2+ and IP3 concentrations. (C) Simulated fast dynamics showing change of membrane potential and Ca2+ concentration.