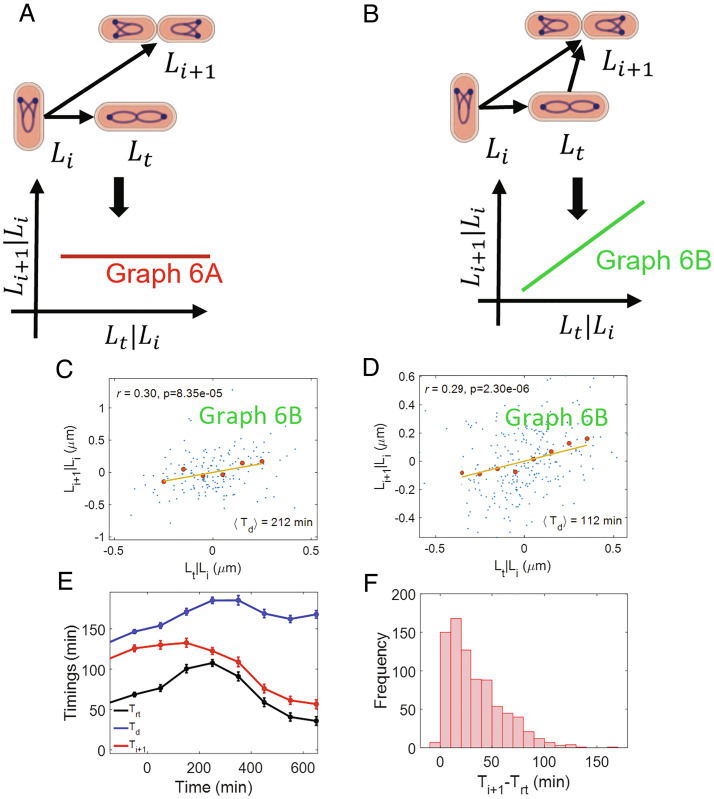
Fig. 6.
Control of replication initiation: (A) A causal graph linking the lengths at initiation per origin (Li), termination (Lt), and the lengths at initiation per origin in the daughter cells (Li + 1). In this graph, the initiation in the daughter cells is solely controlled by initiation in the current cell cycle. We predict r(Lt, Li + 1|Li) =0 for the graph. (B) A causal graph in which the initiation in the daughter cells is controlled simultaneously by initiation and termination in the current cell cycle. We predict a nonzero r(Lt, Li + 1|Li) for the graph. (C and D) Residuals obtained on linear regression of Li + 1 on Li (Li + 1|Li) and Lt on Li (Lt|Li) are plotted for (C) Alanine medium (generation time = 212 min, N = 167 cells, ⟨nori⟩ = 1.08). (D) Glucose medium (generation time = 112 min, N = 255 cells, ⟨nori⟩ = 1.98). The conditional correlation, r(Lt, Li + 1|Li) is nonzero for both alanine and glucose media (consistent with graph 6B). (E and F) ΔthyA cells are grown in a thymine concentration of 500 μ g/mL in M9 glycerol+trace elements medium at 28 °C. The cells are then shifted to a thymine concentration of 15 μ g/mL. Upon shifting to a lower thymine concentration, the C period of the cells increases. We measure the timings at the termination of DNA replication (Trt) and the DNA replication initiation for the next cell cycle (Ti + 1) in multiple cells throughout the experiment (both before and after the shift). (E) Variation of timing (relative to cell birth) of termination, initiation for next cell cycle, and division are plotted. We show the binned data where the cell events’ timings are averaged in each bin based on the time when cell divides (x-axis). Time t = 0 in the x-axis represents the time when cells are shifted to the lower thymine concentration. (F) We plot the distribution of Ti + 1 − Trt timings for all cells measured in the experiment.