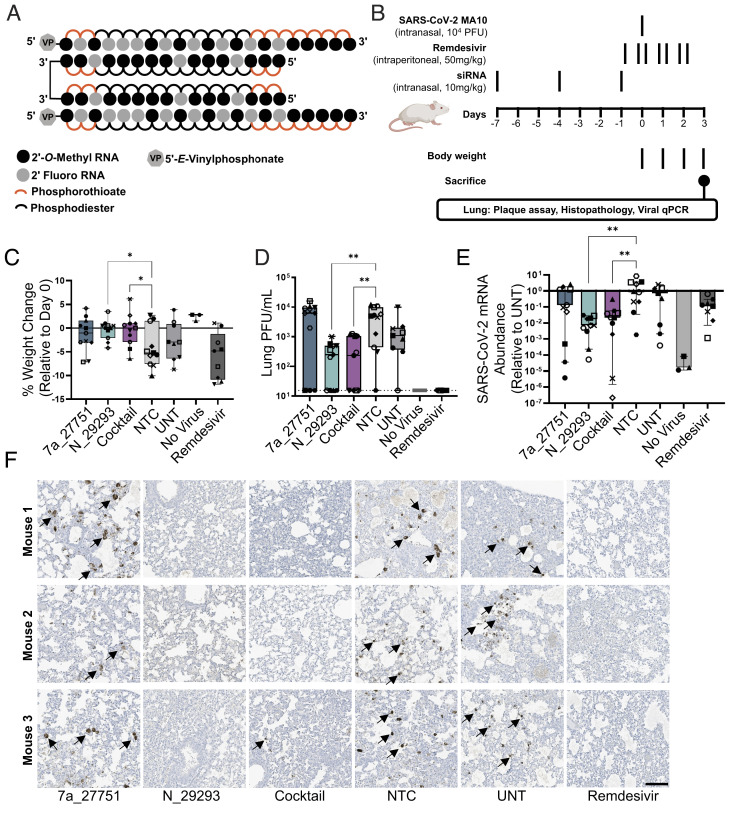
Fig. 5.
Intranasal administration of divalent siRNA is protective in a mouse model of SARS-CoV-2 infection. (A) Schematic of the fully chemically modified divalent siRNA. (B) Study design describing a mouse model of SARS-CoV-2 infection. Divalent siRNAs, 7a_27751, N_29293, and cocktail (1:1 mixture of 7a_27751 and N_29293), were given as pretreatment at days 7, 4, and 1 before viral infection with the SARS-CoV-2 strain MA10 (day 0) by intranasal administration (10 mg/kg/injection). Remdesivir was administered twice a day from day -1 to day 2 by intraperitoneal injection (50 mg/kg/injection) as a positive control. After viral infection, body weight was measured every day, and mice were sacrificed on day 3 (siRNA: n = 11, UNT: n = 9, no virus: n = 3, remdesivir: n = 9 mice). (C) Percent weight change on day 3 relative to day 0 (box plot: center line, median; box limits, upper and lower quartiles; whiskers, min to max; points, independent biological replicates). (D) Viral load in lung quantified by plaque-forming assay (box plot: center line, median; box limits, upper and lower quartiles; whiskers, min to max; points, independent biological replicates). The dotted line indicates the limit of detection (15.6 PFU/mL). (E) Abundance of viral mRNA in lung was measured by qRT-PCR, normalized to a housekeeping gene, Hprt. Data presented relative to UNT (median ± 95% CI of independent biological replicates). (F) Representative immunostaining images of mice lungs. Arrows indicate positive staining of nucleocapsid protein of SARS-CoV-2. Original magnification, ×20. (Scale bar: 100 µm.) C–E, Individual mice are identified by a unique symbol. One-way ANOVA with Dunnett test for multiple comparisons (**P < 0.01, *P < 0.05). MOI, multiplicity of infection; NTC, nontargeting control; UNT, untreated control; PFU, plaque-forming units.