Figure 3. B cells of HCV elite neutralizers encode highly broad and potent bNAbs.
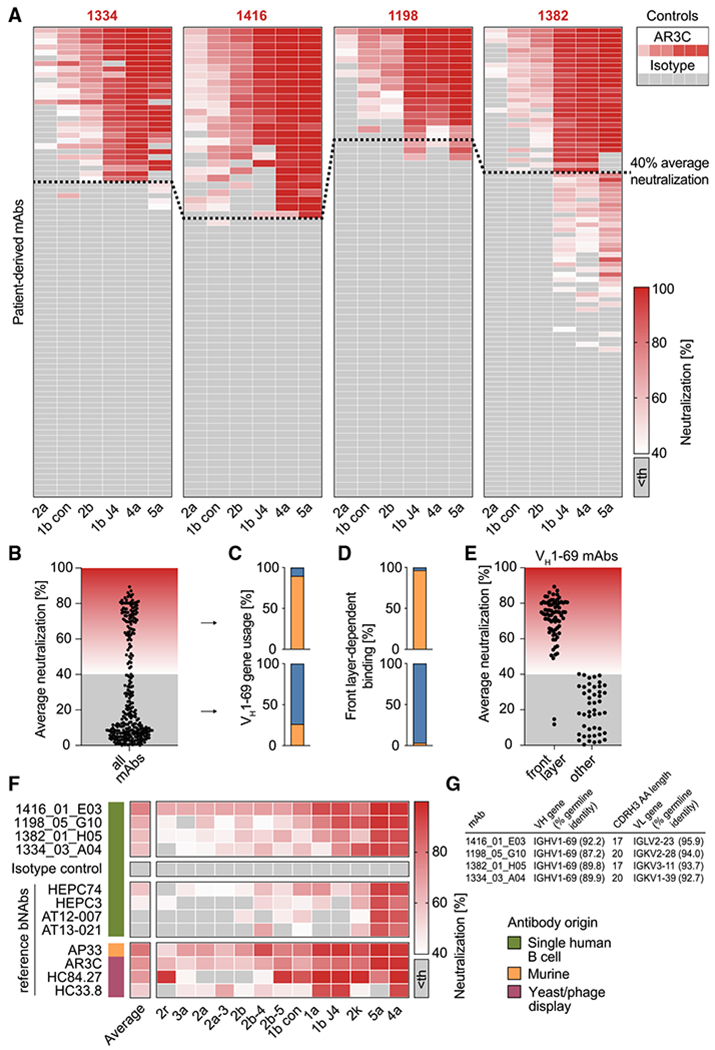
(A) 310 antibodies isolated from HCV elite neutralizers were screened at a concentration of 50 μg/mL for neutralizing activity against HCV as in Figure 1E. Medians of triplicate measurements. Screening was performed once. Antibodies with an average neutralization above 40% (indicated by a dashed line) were defined as potent neutralizers. Th, threshold.
(B) Distribution of average neutralization for all patient-derived antibodies from (A).
(C) Percentage of antibodies utilizing the VH gene segment 1-69 among potent (upper panel) or low/non-neutralizers (lower panel).
(D) Percentage of antibodies binding the E2 protein in a front-layer-dependent manner within all antibodies binding the E2 protein. Determined by ELISA with either wildtype or front layer knockout E2 protein of strain 1a157.
(E) Average neutralization as in (B) for VH1-69 antibodies that bind E2 in either a front layer-dependent or a front layer-independent way.
(F) Four top hits from the neutralization screen in (A) along with a set of reference HCV-specific antibodies were re-tested for neutralizing activity against an extended panel of 13 HCVcc strains, including those used for the first screen. Medians of triplicate measurements, representative of 3 independent experiments.
(G) Genetic properties of the 4 isolated HCV bNAbs assayed in (F). Germline identity is indicated on nucleotide level.
