Abstract
Background:
Hospitalizations among people with dementia (PWD) may precipitate behavioral changes, leading to psychotropic medication use despite adverse outcomes and limited efficacy. We sought to determine the incidence of new psychotropic medication use among community-dwelling PWD after hospital discharge and, among new users, the proportion with prolonged use.
Methods:
This was a retrospective cohort study using a 20% random sample of Medicare claims in 2017, including hospitalized PWD with traditional and Part D Medicare who were 68 years or older. The primary outcome was incident prescribing at discharge of psychotropics including antipsychotics, sedative-hypnotics, antiepileptics, and antidepressants. This was defined as new prescription fills (i.e., from classes not used in 180 days preadmission) within 7 days of hospital or skilled nursing facility discharge. Prolonged use was defined as the proportion of new users who continued to fill newly prescribed medications beyond 90 days of discharge.
Results:
The cohort included 117,022 hospitalized PWD with a mean age of 81 years; 63% were female. Preadmission, 63% were using at least 1 psychotropic medication; 10% were using medications from ≥3 psychotropic classes. These included antidepressants (44% preadmission), antiepileptics (29%), sedative-hypnotics (21%), and antipsychotics (11%). The proportion of PWD discharged from the hospital with new psychotropics ranged from 1.9% (antipsychotics) to 2.9% (antiepileptics); 6.6% had at least one new class started. Among new users, prolonged use ranged from 36% (sedative-hypnotics) to 63% (antidepressants); across drug classes, prolonged use occurred in 51%. Predictors of newly initiated psychotropics included length of stay (≥median vs. <median; aOR 1.5, 95% CI: 1.4–1.6) and delirium (aOR 1.8, 95% CI: 1.7–1.9).
Conclusions:
Hospitalized PWD have a high prevalence of preadmission psychotropic medication use; against this baseline, discharge from the hospital with new psychotropics is relatively uncommon. Nevertheless, prolonged use of newly initiated psychotropics occurs in a substantial proportion of this population.
Keywords: Dementia, hospitalization, psychotropics, prescribing
Introduction
Psychotropic medications are commonly prescribed to people with dementia (PWD) to manage behavioral and psychological symptoms of dementia such as agitation, depression, and apathy. This practice occurs despite a lack of high-quality evidence supporting their use and well-documented harms associated with these medications.1–6 Hospitalizations represent a critical period for PWD and may result from exacerbation of behavioral changes among PWD in the outpatient setting. Given a confluence of transient factors including acute illness, unfamiliar environment, and altered sensory stimuli, hospitalizations themselves may precipitate behavioral changes or delirium in PWD and thereby lead to use of psychotropic medications by inpatient providers.7 Furthermore, medications that are started during hospitalization may be continued upon discharge. This may be due to clinical inertia, perceived need for prolonged use, or lack of specific instructions regarding discontinuation.8,9
The extent to which new psychotropic medications are prescribed to PWD at hospital discharge and patient and hospitalization factors associated with such prescribing are unknown in the United States. Given that hospitalizations among PWD are frequent,10 and that a significant proportion of hospitalized older adults have underlying dementia,11 understanding the role of hospitalizations in potentially increasing psychotropic medication use among PWD is crucial to efforts to reduce long-term use of these medications. To address this evidence gap, we used data on prescription drug claims of a national random sample of Medicare beneficiaries to describe the frequency of newly prescribed psychotropic medications among community-dwelling PWD following medical or surgical hospitalization. We also sought to identify patient and hospitalization factors associated with post-discharge prescribing of these medications. We hypothesized that hospitalizations would be a significant contributor to new initiation of psychotropic medications.
Methods
Study population and data sources:
We identified acute short-term hospitalizations of Medicare beneficiaries admitted from January 1, 2017 to January 1, 2018 using the Centers for Medicare and Medicaid Services MEDPAR (Medicare Provider Analysis and Review) hospital data files for a 20% random sample of beneficiaries (Supplementary Figure S1). We included hospitalizations involving beneficiaries with at least 1 International Classification of Diseases, Ninth Revision, Clinical Modification or International Classification of Diseases, Tenth Revision dementia diagnosis code (Supplementary Table S1) from a health care encounter occurring during the three years preceding hospitalization in line with prior studies.4,12,13 Given the three-year look-back period, we required beneficiaries to be 68 years or older at the start of the accrual period. For beneficiaries who were hospitalized multiple times during the accrual period, we selected one hospitalization at random for analysis.
To improve measurement of medication prescribing, we limited our analyses to beneficiaries who: had continuous Part D prescription drug coverage from at least six months prior to admission to one month after discharge (from either hospital or, if applicable, skilled nursing facility [SNF]); had at least one part D claim in the 6 months prior to admission; and lived through the hospitalization and/or SNF stay and at least seven days following discharge. We excluded beneficiaries enrolled in Medicare Advantage in the year prior to admission given that utilization data may not be complete for these individuals.14 We applied additional criteria to improve our ability to measure prescribing among community-dwelling PWD, excluding hospitalizations in which the beneficiary: 1) transferred to the hospital from another hospital, SNF, hospice, or long-term care facility15; 2) was admitted to a hospital or SNF in the 90 days prior to admission; 3) was discharged to any facility other than SNFs covered by Medicare that are captured in MEDPAR; 4) had a SNF stay following hospital discharge exceeding thirty days; and/or 5) was discharged from the hospital or SNF to hospice (either home or medical facility). Finally, we excluded beneficiaries who were admitted for primary psychiatric reasons, as defined by Agency for Healthcare Research and Quality Clinical Classifications Software codes, in order to focus on hospitalizations for medical and surgical reasons (Supplementary Table S2).16
We linked each hospitalization with beneficiary data from the Medicare Beneficiary Summary File (including age, sex, and dual Medicare-Medicaid eligibility status) and hospitalization characteristics from the MEDPAR file (length of stay, intensive care unit care use). We used categories for race and ethnicity based on the Research Triangle Institute definitions found in Medicare claims.17 As a validated measure incorporating 30 comorbidities, we calculated the Elixhauser Comorbidity Index (van Walraven adaption) for each beneficiary.18,19 We determined the rurality status of each beneficiary using the 2013 National Center for Health Statistics Urban-Rural Classification Scheme for Counties. We categorized large central metropolitan, large fringe metropolitan, medium metropolitan, and small metropolitan counties as urban and micropolitan and non-core counties as rural.20 We categorized reasons for hospitalization as medical or surgical based on major diagnostic categories in line with a prior study.21 We identified delirium during the hospitalization based on a previously described ICD claims-based algorithm (Supplementary Table S3).22 We determined whether patients were discharged to home versus SNF with the MEDPAR file: those with claims for a SNF admission on the day of, or the day after, hospital discharge were classified as discharge to SNF, and others were classified as discharge to home (other discharge destinations were excluded as described above).23
Outcomes and Statistical Analysis:
Medicare Part D data can shed light on prescribing decisions at discharge since new outpatient prescription fills within a few days of discharge most likely represent continuation of medications started during the peri-hospitalization period.21 We estimated medication exposure using Part D prescription fills, relying on the Medi-Span (Wolters Kluwer) crosslink system to identify medications.24 We defined preadmission medications as those with fills of 28 days or more in the 180 days prior to admission. This approach allows for missed pills or late refills as well as identification of as-needed use, and was found to have a sensitivity of 93%, specificity of 97%, and positive predictive value of 89% in identifying use of common chronic medications compared to a criterion standard of medication reconciliation notes.25,26 Using this approach, we calculated a preadmission medication count for each beneficiary, inclusive of all medications (not just psychotropic medications). To provide context for the number of beneficiaries using relevant medications for shorter periods of time, we additionally calculated the number of beneficiaries who filled prescriptions across psychotropic drug classes (described below) for fewer than 28 days in the 180 days prior to admission.
The primary outcome was incident prescribing at discharge of psychotropic medications from the following 4 classes: antidepressants, antipsychotics, antiepileptics, and sedative-hypnotics (including benzodiazepines and nonbenzodiazepine benzodiazepine receptor agonists or “Z-drugs”). The medications included in each of these categories are in Supplementary Table S4. Due to the absence of Part D claims during SNF stays, for PWD who had a SNF stay following hospitalization, we defined post-discharge medication use as drugs received following discharge from SNF. As such, we determined incident prescribing based on fills between one day before and seven days after discharge from the hospital or SNF for drug classes that were not used prior to the hospitalization. We selected these drug classes because they are commonly used off-label in the management of the behavioral symptoms of dementia and are clinically significant contributors to central nervous system (CNS)-active polypharmacy in older adults.6 We used a composite outcome indicating the initiation of at least one new class of psychotropic medication from among the four classes. If a beneficiary was taking a medication within one psychotropic drug class (e.g., an antidepressant) prior to admission and was prescribed a different medication within that same class after discharge, this new medication was not counted as an incident prescription. This is because our focus was on initiation of new psychotropic drug classes. A secondary outcome was prolonged use of medications, defined as the proportion of new users who continued to fill newly prescribed drugs within 90–180 days of discharge (among those who continued to have Part D coverage and were alive during this period) in line with other studies.27–29 Beneficiaries did not necessarily have to have a prescription filled continuously from days 7–90 to be included in the prolonged use group. We performed a stratified analysis by discharge disposition given important clinical differences between patients returning home versus requiring SNF-level care, comparing prescribing outcomes between SNF and home discharges using chi-squared tests.
In an exploratory analysis, we assessed associations between clinically relevant patient and hospitalization factors measurable in administrative claims and the composite outcome of incident prescribing post-hospitalization of at least one psychotropic drug class. We examined relationships with multilevel multivariable mixed effect logistic regression models with adjustment for all the measured patient and hospitalization characteristics and inclusion of random intercepts for each hospital to account for clustering of care within hospitals. As missing data were extremely uncommon, with the highest rate in the rurality variable (0.2%), we used a complete case approach to regression analyses. We used SAS, version 9.4 (SAS Institute, Inc.).
This study was approved by the institutional review board of the University of California, San Francisco and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Results
Sample characteristics:
Our sample included 117,022 hospitalized PWD, whose characteristics are shown in Table 1. These individuals had a mean age of 81 years, and 63% were female. Race/ethnicity was distributed as follows: non-Hispanic White (81%), non-Hispanic Black (9%), Hispanic (6%), and Other (4%). Prior to hospitalization, 63% were using at least 1 psychotropic medication; 10% were using medications from three or more psychotropic classes. These included antidepressants (used by 44% of PWD prior to admission), antiepileptics (29%, the majority of which were gabapentinoids), sedative-hypnotics (21%), and antipsychotics (11%). The median length of stay for hospitalizations was 3 days; 11% of hospitalizations involved ICU stay, 13% had delirium noted as a discharge diagnosis, and 14% involved discharge to SNF billed to Medicare. Supplementary Table S5 shows a comparison of relevant characteristics between those beneficiaries who were discharged to SNF and those discharged to home.
Table 1:
Cohort Characteristics
| Characteristic | Hospitalized Medicare beneficiaries with dementia N = 117,022 |
|---|---|
| Patient factors | |
| Age, years, Mean (SD) | 81.0 (7.7) |
| 68–75 | 33187 (28%) |
| 76–85 | 48194 (41%) |
| ≥86 | 35641 (31%) |
| Sex | |
| Female | 73961 (63%) |
| Male | 43061 (37%) |
| Race/ethnicity | |
| Non-Hispanic White | 94565 (81%) |
| Non-Hispanic Black | 10666 (9%) |
| Hispanic | 7366 (6%) |
| Other | 4425 (4%) |
| Medicaid dual eligibility | |
| Yes | 37364 (32%) |
| No | 79658 (68%) |
| Rurality status | |
| Rural | 25207 (22%) |
| Urban | 91635 (78%) |
| Elixhauser comorbidity score, Median (IQR) | 16 (9, 24) |
| Preadmission medication count, Median (IQR) | 8 (5, 11) |
| Preadmission psychotropic medication use (% of beneficiaries using at least one drug within each category or sub-category) | |
| Antipsychotics | 13042 (11%) |
| “Z-drugs” | 4305 (4%) |
| Antidepressants | 51879 (44%) |
| Other Antiepileptics | 14895 (13%) |
| Preadmission number of psychotropic drug classes | |
| None | 43199 (37%) |
| 1 | 38643 (33%) |
| 2 | 23490 (20%) |
| 3 | 9652 (8%) |
| 4 | 2038 (2%) |
| Hospitalization factors | |
| Length of stay, days, Median (IQR) | 3 (2, 5) |
| ICU stay as part of hospitalization | 12383 (11%) |
| Discharge diagnosis of delirium | 15654 (13%) |
| Discharge destination | |
| Skilled nursing facility | 16915 (14%) |
| Home | 100107 (86%) |
| Reason for hospitalization | |
| Medical | 92563 (79%) |
| Surgical | 24452 (21%) |
For Medicare beneficiaries with dementia with multiple hospitalizations during the study period, we selected one admission at random for analysis. For race/ethnicity, the Other category includes beneficiaries with race/ethnicity of Unknown, Asian/Pacific Islander, and American Indian/Alaska Native. “Z-drugs” refers to non-benzodiazepine, benzodiazepine receptor agonists. The preadmission medication count includes all medication categories (not just psychotropic medications). The preadmission psychotropic medication use rows refer to individuals taking at least one drug from each category. Percentages are rounded to the nearest integer and may not sum to one hundred percent for each category as a result.
Discharge from hospital with new psychotropic medications and prolonged use:
Figure 1 depicts new post-discharge use and prolonged use of psychotropic medications across drug classes. The proportion of PWD discharged from the hospital with a new psychotropic medication ranged from 1.9% (antipsychotics) to 2.9% (antiepileptics); 6.6% had at least one new class started. Prolonged use of newly prescribed medication classes was considerably higher across all drug classes, ranging from 36% (sedative-hypnotics) to 55% (antipsychotics) to 63% (antidepressants); across drug classes, prolonged use occurred in 51%. Among those with prolonged use of newly prescribed medications, the median number of days supplied for prescriptions in the 180 days following discharge ranged from 100 days for sedative-hypnotics to 202 days for antidepressants (Supplementary Table S6). The analysis stratified by discharge destination (Supplementary Tables S7 and S8) revealed that both new post-discharge prescribing and prolonged use occurred at a greater rate in those discharged to SNF compared to home (at least one new class was started in 8.7% discharged to SNF vs. 6.2% discharged to home [P<0.001], and among those who initiated, 57.8% vs. 49.4% had prolonged use, respectively [P<0.001]). Among beneficiaries who were not taking any psychotropic medications prior to admission, the proportion discharged from the hospital with a new psychotropic medication was similar to the main analysis across drug classes (Supplementary Table S9). Supplementary Table S10 shows the number of beneficiaries who filled prescriptions across psychotropic drug classes for fewer than 28 days in the 180 days prior to admission.
Figure 1: Incident post-discharge use and prolonged use of psychotropic drugs among hospitalized older adults with dementia, with exemplar flow diagram depicting antipsychotic use.
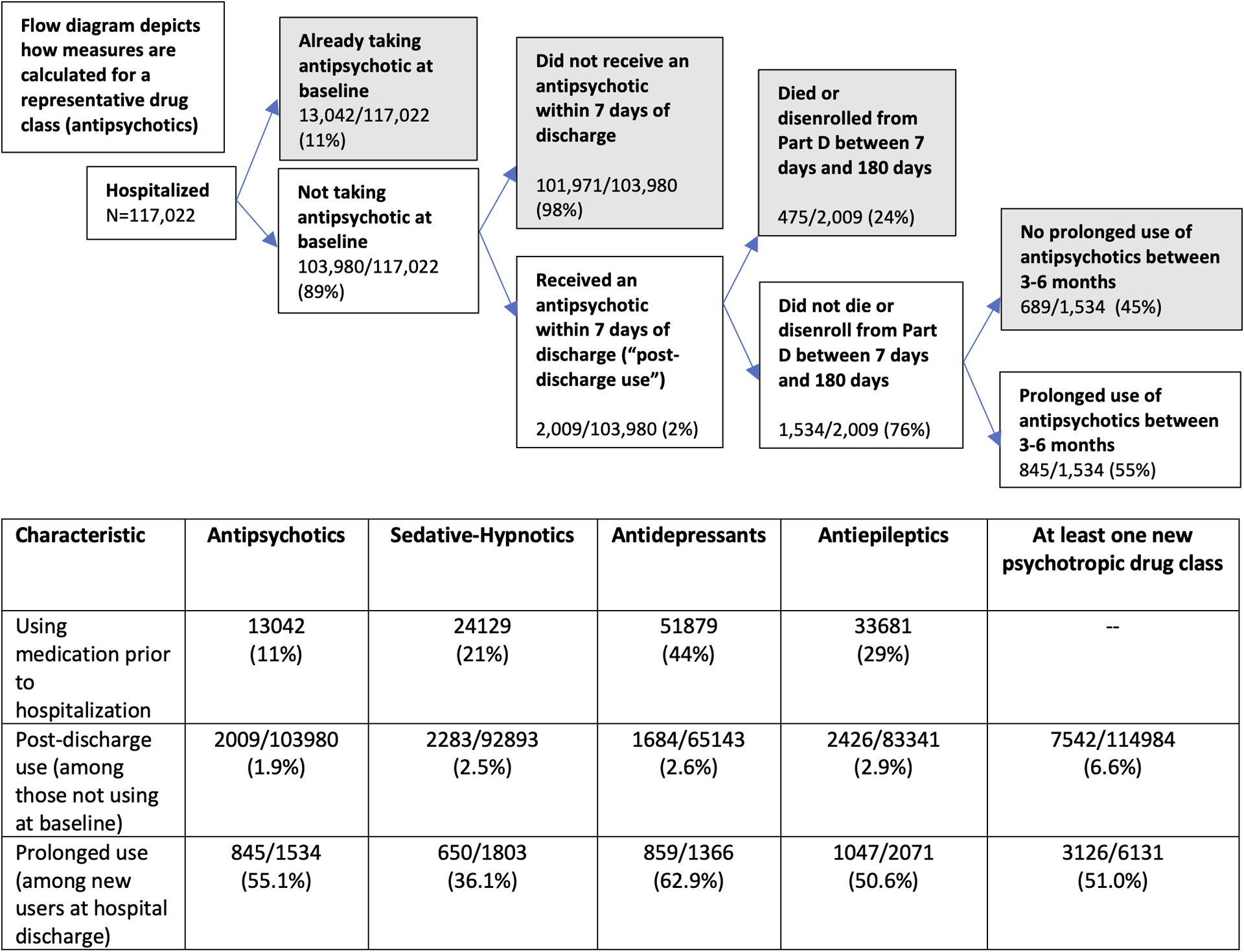
The flow diagram shows how measures are calculated for a representative drug class (antipsychotics); all prescribing outcomes are included in the accompanying table. For the prolonged use measure, the denominator excludes individuals who either died before or during the 90–180 day range following hospital discharge or did not have continuous Part D insurance during this period.
Patient and hospital factors associated with post-discharge prescribing:
Associations between patient and hospital factors and post-discharge prescribing of at least one new psychotropic class are shown in Table 2. After adjustment for all measured patient and hospitalization factors, three patient factors were each associated with a lower odds of post-discharge prescribing of a new psychotropic: use of ≥5 medications prior to admission (adjusted odds ratio [aOR]: 0.70, 95% confidence interval [CI]: 0.66–0.74, compared with <5 medications), higher comorbidity burden (Elixhauser score, 2nd quartile [Q2] vs. Q1, aOR: 0.87, 95% CI: 0.81–0.93; Q3 vs. Q1, aOR: 0.77, 95% CI: 0.72–0.82; Q4 vs Q1, aOR 0.75, 95% CI: 0.70–0.80), and non-White race/ethnicity (non-Hispanic Black vs. non-Hispanic White, aOR: 0.90, 95% CI: 0.82–0.98). Among hospitalization factors, significant predictors included discharge diagnosis of delirium (aOR 1.76, 95% CI: 1.66–1.87); longer length of stay (≥3 days vs. <3 days, aOR 1.50, 95% CI: 1.42–1.59); discharge to SNF (compared to home; aOR 1.18, 95% CI: 1.11–1.26); ICU stay (aOR 1.14, 95% CI: 1.06–1.23); and reason for hospitalization (surgical vs. medical, aOR 0.81, 95% CI: 0.76–0.87).
Table 2:
Associations between patient and hospitalization factors and post-discharge prescribing of at least one new psychotropic drug class
| Characteristic | Unadjusted Associations | Adjusted Associations | ||
|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | P value | Odds Ratio (95% Confidence Interval) | P value | |
| Patient factors | ||||
| Age, years [ref: 68–75 years] | 0.09 | 0.14 | ||
| 76–85 | 1.05 (0.99, 1.11) | 1.00 (0.94, 1.06) | ||
| ≥86 | 1.07 (1.01, 1.14) | 0.95 (0.89, 1.01) | ||
| Female sex | 1.05 (1.00, 1.10) | 0.04 | 1.02 (0.97, 1.07) | 0.44 |
| Race/ethnicity [ref: Non-Hispanic White] | 0.006 | 0.01 | ||
| Non-Hispanic Black | 0.91 (0.83, 0.99) | 0.90 (0.82, 0.98) | ||
| Hispanic | 0.89 (0.80, 0.99) | 0.90 (0.80, 1.00) | ||
| Other | 0.88 (0.77, 1.00) | 0.88 (0.77, 1.01) | ||
| Medicaid dual eligible status | 0.97 (0.92, 1.02) | 0.22 | 1.00 (0.94, 1.06) | 0.97 |
| Urban living status | 1.04 (0.98, 1.10) | 0.25 | 1.01 (0.95, 1.07) | 0.78 |
| Elixhauser comorbidity score [ref: Quartile 1] | <0.001 | <0.001 | ||
| Quartile 2 | 0.94 (0.88, 1.00) | 0.87 (0.81, 0.93) | ||
| Quartile 3 | 0.84 (0.79, 0.90) | 0.77 (0.72, 0.82) | ||
| Quartile 4 | 0.83 (0.78, 0.89) | 0.75 (0.70, 0.80) | ||
| ≥5 medications used at baseline | 0.69 (0.65, 0.72) | <0.001 | 0.70 (0.66, 0.74) | <0.001 |
| Hospitalization factors | ||||
| Length of stay for index hospitalization ≥median (3 days) | 1.59 (1.50, 1.67) | <0.001 | 1.50 (1.42, 1.59) | <0.001 |
| ICU stay as part of hospitalization | 1.17 (1.08, 1.25) | <0.001 | 1.14 (1.06, 1.23) | <0.001 |
| Discharge diagnosis of delirium | 1.87 (1.76, 1.98) | <0.001 | 1.76 (1.66, 1.87) | <0.001 |
| Discharge to skilled nursing facility [ref: Home] | 1.43 (1.35, 1.52) | <0.001 | 1.18 (1.11, 1.26) | <0.001 |
| Surgical reason for hospitalization [ref: Medical] | 0.85 (0.80, 0.90) | <0.001 | 0.81 (0.76, 0.87) | <0.001 |
The results reflect multilevel mixed-effect logistic regression models, with random intercepts for each hospital and, in adjusted analyses, with inclusion of all listed variables.
Discussion
In a nationally representative sample of Medicare beneficiaries hospitalized for medical or surgical reasons, community-dwelling older adults with dementia had a strikingly high degree of baseline psychotropic medication use, while a relatively small proportion—6.6%—were prescribed at least one new class of psychotropic medications after discharge. Of these newly prescribed psychotropic medications, a significant proportion—half—were still in use three to six months after discharge. In exploratory analyses, we identified several patient and hospitalization factors associated with post-discharge prescribing of psychotropic medications. These findings suggest hospitalizations likely play a relatively small role in adding to the overall burden of psychotropic medications taken by community-dwelling PWD, and that efforts to address new prescribing of such medications among people with dementia may be better targeted to other healthcare settings. Nevertheless, it should not be overlooked that prolonged use of newly initiated psychotropics occurs in a substantial proportion of this population. Additionally, given the high prevalence of CNS polypharmacy prior to admission, hospitalizations may represent a time to deprescribe potentially unnecessary or harmful medications in PWD under the right clinical circumstances.30
Several recent European studies have examined the role of hospitalizations in prescribing of psychotropic medications among PWD.31–34 Möllers et al. examined this question among beneficiaries with dementia enrolled in a German health program, finding new post-hospital use of psychotropic medications ranging from 1.8% (antidepressants) to 7.1% (antipsychotics).32 However, the adjudication period for new prescribing was up to 30 days following discharge in this study, compared to a 7-day window in our study, which provides a more conservative estimate for prescriptions that were started during the hospitalization and continued upon discharge. Reinold et al. conducted a cohort study of older adults with dementia in a single Italian hospital, finding that the Anticholinergic Cognitive Burden score was higher at discharge compared to preadmission; this was attributed to an increase in prevalent prescribing of antipsychotics with anticholinergic activity from 12% prior to admission to 33% at the time of discharge.31 In the US, a handful of studies have focused specifically on the use of antipsychotics during non-psychiatric hospitalizations. As opposed to our focus on PWD, these studies focused on adults generally35,36 and on older cardiac surgery patients,37 finding that dementia was a risk factor for new in-hospital prescribing of antipsychotics and potentially excessive doses of these medications.
A striking finding of our study was that many PWD were already taking many psychotropic medications preadmission, with 63% using at least 1 psychotropic medication and one in ten using medications from three or more psychotropic classes prior to admission. This latter finding dovetails with a recent analysis of Medicare beneficiaries that found a prevalence of almost 14% of CNS-active polypharmacy among PWD (with opioids included in their definition).4 Against this high baseline prevalence, we found a relatively modest incidence of new prescribing of psychotropics on discharge, in the range of 2–3% per medication class. This analysis focused on a single hospitalization during a one-year sampling frame; PWD may nevertheless be readmitted over time, and repeated hospitalizations could lead to the accrual of more medications. We found that the prescribing rates among hospitalized PWD without any preadmission psychotropic drug use were similarly modest across drug classes compared to those in the entire cohort. This finding lends credence to the suggestion that hospitalizations do not play an outsized role in contributing to the striking burden of psychotropic medications in this population. Overall, while we were unable to discern the indication for prescribing these medications in the hospital, it is likely that a significant proportion were being used for behavioral and psychological symptoms of dementia such as depression, agitation, or apathy. The relatively low rates may be consistent with providers avoiding the use of these medications in PWD, or it may be that there was little room for addition of new psychotropic drug classes given the high preadmission prevalence.
We found that half of PWD who were prescribed a new psychotropic medication during hospitalization or around the time of discharge continued to use these medications on a prolonged basis. Additionally, while we defined prolonged use based on a 90-day timeframe, many beneficiaries whose prescriptions did not reach this threshold were nevertheless exposed to varying degrees of persistent use in the peri-hospitalization period (Supplementary Table S6). Among PWD, antidepressants used for depression and potentially for other neuropsychiatric symptoms of dementia such as agitation have demonstrated limited efficacy, but this must be weighed against the potential harms of therapy (e.g., prolongation of the QT interval in those taking citalopram).1,2,33 Sedative agents such as benzodiazepines carry serious risk of adverse events in older adults—especially those with cognitive impairment—with little evidence of efficacy for behavioral symptoms1,2; as such, prolonged use of these agents among over one-third of new post-discharge users in our sample is a cause for concern. Similarly, while antipsychotics are sometimes used for the management of severe behavioral symptoms that pose safety risks, they are associated with myriad adverse events in PWD including sedation, cognitive decline, cardiac arrhythmia, and even death.1,2,39 Furthermore, consensus guidelines recommend limiting use of these medications to the shortest time period possible, with attempts at tapering and discontinuation over time.6,40 These findings raise a concern that at least some psychotropic medications started during hospitalization or around the time of discharge for PWD contribute to prolonged use that is potentially inappropriate and lacking a clear indication.
Some of the associations we observed in the exploratory analysis linking patient and hospitalization factors to incident psychotropic prescribing were unexpected. Multiple coexisting medical illnesses and polypharmacy are associated with delirium in older adults,6,41,42 which in turn is associated with off-label antipsychotic initiation during acute hospitalization.36 In this light, the reason that an increasing Elixhauser comorbidity score and taking a greater number of preadmission medications were each associated with a lower odds of receiving a new psychotropic medication at time of discharge is not immediately apparent. One possible explanation is that individuals with greater comorbidity burden and polypharmacy were less likely to receive a new psychotropic drug given the fact that they may have already been taking one or more psychotropic drugs at baseline and possible awareness about the risks of CNS-active polypharmacy. The findings that PWD with longer lengths of stay, ICU stays, discharge diagnosis of delirium, and discharge to SNF were more likely to receive new psychotropic drugs post-discharge support the notion that especially long or complicated hospitalizations, or those involving SNF discharge, may represent appropriate targets for programs seeking to contain new initiation of psychotropic drugs during hospitalization or around the time of discharge.
There are several limitations to consider in interpreting our study. First, cohort identification relied on a dementia diagnosis in claims data; while this approach confers reasonable accuracy in capturing dementia diagnoses,13 it is likely that some individuals with dementia were not identified, while some individuals were identified who did not actually have dementia. Second, given the nature of Medicare prescription claims, we were unable to observe prescribing during hospitalizations and SNF stays, and therefore to know when precisely during the peri-hospitalization period the decision was made to start medications. Our primary outcome was incident prescribing within seven days following discharge from the hospital or SNF of psychotropic medications. It is possible that this may reflect de novo outpatient prescribing of these medications rather than continuation of medications started during the acute hospitalization or SNF stay, but this is unlikely given the relatively short time frame.21 Additionally, we were unable to discern decisions to deprescribe the psychotropic medications under study during the post-hospitalization period. Third, while the finding that delirium was associated with a significantly greater odds of receiving a new post-discharge psychotropic medication is clinically intuitive, identification of delirium solely using administrative claims is known to have low sensitivity.22 Therefore, we likely underestimated the true prevalence of delirium in the study sample; the noted association most likely speaks to a linkage of severe or hyperactive delirium with new prescribing of psychotropics. Fourth, our study was not designed to determine the accumulation over time of psychotropic medication initiation across multiple hospitalizations or the possible variation in per hospitalization initiation of psychotropic medications across classes. Additionally, we did not examine new or different medications initiated within a given psychotropic drug class if a beneficiary was already taking a drug from that class prior to admission. Finally, these findings may not be generalizable to Medicare Advantage beneficiaries with dementia.
In conclusion, community-dwelling older adults with dementia who are hospitalized have a high prevalence of preadmission psychotropic medication use; against this baseline, hospital discharge with new psychotropic medications is relatively uncommon. Nevertheless, prolonged use of newly initiated psychotropic drugs occurs in a substantial proportion of this population. This underscores the importance of continually assessing the potential benefits and harms of medications started during hospitalization or around the time of discharge.
Supplementary Material
Key points:
In this nationally representative cohort of 117,022 hospitalized older Medicare beneficiaries with dementia, 63% were using at least 1 psychotropic medication preadmission.
Discharge from the hospital with at least one new psychotropic drug class occurred in 6.6% (7,542/114,984).
Among new users of these medications at discharge, half continued to use them at 3–6 months.
Why does this matter?
Acute hospitalizations likely play a relatively small role in adding to psychotropic medications taken by community-dwelling people with dementia (PWD). Nevertheless, prolonged use of hospital-initiated psychotropic medications is common. This finding raises the concern that some psychotropic medications started during hospitalization or around the time of discharge for PWD contribute to prolonged use that is potentially inappropriate and lacking a clear indication.
ACKNOWLEDGEMENTS
Conflict of interest statement:
Dr. Steinman received personal fees from UpToDate Royalties for authoring a chapter in UpToDate and personal fees from American Geriatrics Society Honoraria for serving as co-chair of the American Geriatrics Society Beers Criteria Guideline Panel outside the submitted work. The authors have no other disclosures to report.
Role of the Sponsor:
The National Institute on Aging and the National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding/support:
The following authors were supported by the National Institute on Aging (NIA): Dr Growdon (T32-AG000212, R03AG078804), Dr. Yaffe (R35AG071916, P01AG066605), Dr. Lee (K01AG073532), Dr. Anderson (R03AG064373), and Dr. Steinman (P30AG044281, 1R24AG064025, K24AG049057, P01AG066605). Dr. Growdon was also supported by the following grant from NIH/AHRQ: K12HS026383.
Footnotes
Previous Presentation: This project was accepted in abstract form for the American Geriatrics Society Annual Scientific Meeting in Orlando, FL (May 13, 2022).
References
- 1.Sink KM, Holden KF, Yaffe K. Pharmacological Treatment of Neuropsychiatric Symptoms of Dementia: A Review of the Evidence. JAMA. 2005;293(5):596. doi: 10.1001/jama.293.5.596 [DOI] [PubMed] [Google Scholar]
- 2.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. The BMJ. 2015;350. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maust DT, Strominger J, Bynum JPW, et al. Prevalence of Psychotropic and Opioid Prescription Fills Among Community-Dwelling Older Adults With Dementia in the US. JAMA. 2020;324(7):706–708. doi: 10.1001/jama.2020.8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maust DT, Strominger J, Kim HM, et al. Prevalence of Central Nervous System–Active Polypharmacy Among Older Adults With Dementia in the US. JAMA. 2021;325(10):952. doi: 10.1001/jama.2021.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Growdon ME, Gan S, Yaffe K, Steinman MA. Polypharmacy among older adults with dementia compared with those without dementia in the United States. J Am Geriatr Soc. 2021;69(9):2464–2475. doi: 10.1111/jgs.17291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 7.Gallagher P, Curtin D, de Siún A, et al. Antipsychotic prescription amongst hospitalized patients with dementia. QJM Int J Med. 2016;109(9):589–593. doi: 10.1093/qjmed/hcw023 [DOI] [PubMed] [Google Scholar]
- 8.Scales DC, Fischer HD, Li P, et al. Unintentional Continuation of Medications Intended for Acute Illness After Hospital Discharge: A Population-Based Cohort Study. J Gen Intern Med. 2016;31(2):196–202. doi: 10.1007/s11606-015-3501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge Plans for Geriatric Inpatients with Delirium: A Plan to Stop Antipsychotics? J Am Geriatr Soc. 2017;65(10):2278–2281. doi: 10.1111/jgs.15026 [DOI] [PubMed] [Google Scholar]
- 10.Anderson TS, Marcantonio ER, McCarthy EP, Herzig SJ. National Trends in Potentially Preventable Hospitalizations of Older Adults with Dementia. J Am Geriatr Soc. 2020;68(10):2240–2248. doi: 10.1111/jgs.16636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmons S, Manning E, Barrett A, et al. Dementia in older people admitted to hospital: a regional multi-hospital observational study of prevalence, associations and case recognition. Age Ageing. 2015;44(6):993–999. doi: 10.1093/ageing/afv131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Wilson L, Kornak J, et al. The costs of dementia subtypes to California Medicare fee-for-service, 2015. Alzheimers Dement. 2019;15(7):899–906. doi: 10.1016/j.jalz.2019.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moura LMVR, Festa N, Price M, et al. Identifying Medicare beneficiaries with dementia. J Am Geriatr Soc. n/a(n/a). doi: 10.1111/jgs.17183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Identifying Medicare Managed Care Beneficiaries from the Master Beneficiary Summary or Denominator Files | ResDAC. Accessed March 15, 2022. https://resdac.org/articles/identifying-medicare-managed-care-beneficiaries-master-beneficiary-summary-or-denominator [Google Scholar]
- 15.Prabaker KK, Hayden MK, Weinstein RA, Lin MY. Use of the point of origin code from a universal billing form, UB-04, to efficiently identify hospitalized patients admitted from other health care facilities. Am J Infect Control. 2012;40(7):659–662. doi: 10.1016/j.ajic.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Healthcare Cost and Utilization Project (HCUP) SID Notes. Accessed May 3, 2022. https://www.hcup-us.ahrq.gov/db/vars/siddistnote.jsp?var=i10_serviceline
- 17.Jarrín OF, Nyandege AN, Grafova IB, Dong X, Lin H. Validity of Race and Ethnicity Codes in Medicare Administrative Data Compared With Gold-standard Self-reported Race Collected During Routine Home Health Care Visits. Med Care. 2020;58(1):e1. doi: 10.1097/MLR.0000000000001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A Modification of the Elixhauser Comorbidity Measures Into a Point System for Hospital Death Using Administrative Data. Med Care. 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5 [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data: Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 20.Ingram D, Franco S. 2013 NCHS urban–rural classification scheme for counties. National Center for Health Statistics. Vital Health Stat. 2014;2(166). [PubMed] [Google Scholar]
- 21.Jena AB, Goldman D, Karaca-Mandic P. Hospital Prescribing of Opioids to Medicare Beneficiaries. JAMA Intern Med. 2016;176(7):990–997. doi: 10.1001/jamainternmed.2016.2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Lee J, Kim CA, et al. Evaluation of Algorithms to Identify Delirium in Administrative Claims and Drug Utilization Database. Pharmacoepidemiol Drug Saf. 2017;26(8):945–953. doi: 10.1002/pds.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Grunditz J, Meath T, Quiñones A, Ibrahim S, McConnell K. Accuracy of Hospital Discharge Codes in Medicare Claims for Knee and Hip Replacement Patients. Med Care. 2020;58(5):491–495. doi: 10.1097/MLR.0000000000001290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medi-Span: Drug Data Solutions for Healthcare. Accessed March 17, 2022. https://www.wolterskluwer.com/en/solutions/medi-span
- 25.Anderson TS, Xu E, Whitaker E, Steinman MA. A systematic review of methods for determining cross-sectional active medications using pharmacy databases. Pharmacoepidemiol Drug Saf. 2019;28(4):403–421. doi: 10.1002/pds.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson TS, Jing B, Wray CM, et al. Comparison of pharmacy database methods for determining prevalent chronic medication use. Med Care. 2019;57(10):836–842. doi: 10.1097/MLR.0000000000001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bongiovanni T, Gan S, Finlayson E, et al. Prolonged use of newly prescribed gabapentin after surgery. J Am Geriatr Soc. Published online 2022:1–10. doi: 10.1111/jgs.18005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. doi: 10.1136/bmj.l1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brummett CM, Waljee J, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgery in U.S. Adults. JAMA Surg. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam K, Rochon PA, Steinman MA. Often Off-label: Questionable Gabapentinoid Use Noted at Hospital Admission Warrants Deprescribing. J Hosp Med. 2019;14(9):579–580. doi: 10.12788/jhm.3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinold J, Palese F, Romanese F, Logroscino G, Riedel O, Pisa FE. Anticholinergic burden before and after hospitalization in older adults with dementia: Increase due to antipsychotic medications. Int J Geriatr Psychiatry. 2019;34(6):868–880. doi: 10.1002/gps.5084 [DOI] [PubMed] [Google Scholar]
- 32.Möllers T, Perna L, Stocker H, et al. New use of psychotropic medication after hospitalization among people with dementia. Int J Geriatr Psychiatry. 2020;35(6):640–649. doi: 10.1002/gps.5282 [DOI] [PubMed] [Google Scholar]
- 33.Hakala A, Tolppanen AM, Koponen M, et al. Does Recent Hospitalization Increase Antipsychotic Initiation Among Community Dwellers With Alzheimer’s Disease? J Am Med Dir Assoc. 2021;22(7):1543–1547.e3. doi: 10.1016/j.jamda.2020.12.021 [DOI] [PubMed] [Google Scholar]
- 34.Tarvainen A, Hartikainen S, Taipale H, Tanskanen A, Koponen M, Tolppanen AM. Association of recent hospitalisation with antidepressant initiation among community dwellers with Alzheimer’s disease. Int J Geriatr Psychiatry. 2021;36(7):1075–1084. doi: 10.1002/gps.5505 [DOI] [PubMed] [Google Scholar]
- 35.Herzig SJ, Rothberg MB, Guess JR, et al. Antipsychotic Use in Hospitalized Patients: Rates, Indications, and Predictors. J Am Geriatr Soc. 2016;64(2):299–305. doi: 10.1111/jgs.13943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med. 2016;11(8):543–549. doi: 10.1002/jhm.2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DH, Mahesri M, Bateman BT, et al. Longitudinal Trends and Variation in Antipsychotic Use in Older Patients After Cardiac Surgery. J Am Geriatr Soc. 2018;66(8):1491–1498. doi: 10.1111/jgs.15418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–691. doi: 10.1001/jama.2014.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438–445. doi: 10.1001/jamapsychiatry.2014.3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia. Am J Psychiatry. 2016;173(5):543–546. doi: 10.1176/appi.ajp.2015.173501 [DOI] [PubMed] [Google Scholar]
- 41.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in Older Persons. JAMA. 2017;318(12):1161–1174. doi: 10.1001/jama.2017.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hein C, Forgues A, Piau A, Sommet A, Vellas B, Nourhashémi F. Impact of Polypharmacy on Occurrence of Delirium in Elderly Emergency Patients. J Am Med Dir Assoc. 2014;15(11):850.e11–850.e15. doi: 10.1016/j.jamda.2014.08.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


