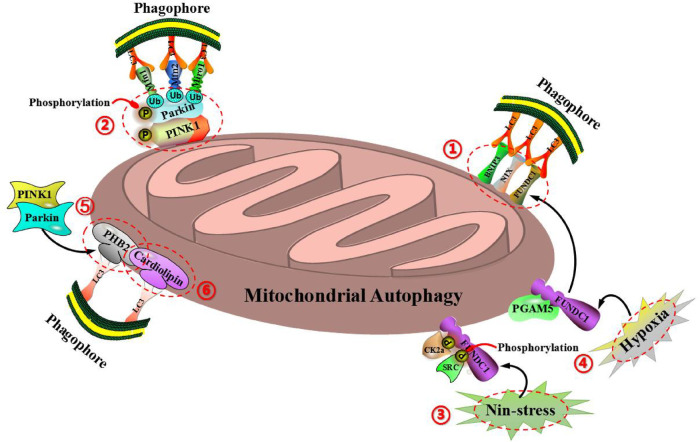
Figure 3.
Molecular mechanism of mitochondrial autophagy regulation can be divided into two types: ubiquitin-dependent mitochondrial autophagy (including PINK1 and Parkin) and ubiquitin-independent mitochondrial autophagy (including BNIP3, NIX, FUNDC1, PHB2, cardiolipin and so on). (1) BNIP3, FUNDC1, and NIX are all located on the outer membrane of mitochondria and can bind directly to LC3-mediated mitochondrial autophagy via the domain of LC3 interaction (LIR). (2) BNIP3 can also inhibit the proteolysis of PINK1, so as to cause the accumulation of PINK1 on the outer mitochondrial membrane, thereby promoting PINK1/Parkin-mediated mitochondrial autophagy. (3) Under the condition of non-stress, FUNDC1 is phosphorylated by protein kinase CK2α in the region of Ser-13 and phosphorylated by protein kinase SRC in the region of Tyr-18, so as to inhibit the interaction of LC3 with FUNDC1, thereby preventing the occurrence of mitochondrial autophagy. (4) Under the condition of mitochondrial membrane potential loss or hypoxia, protein phosphatase PGAM5 can interact with FUNDC1 to prevent CK2α and SRC kinase to combin with FUNDC1, resulting in the dephosphorylation of FUNDC1, so as to enhance the interaction between FUNDC1 and LC3, and induce mitochondrial autophagy. (5) As an important receptor for mitochondrial autophagy, the inner mitochondrial membrane protein PHB2 can mediate mitochondrial autophagy via the domain of LIR, and bind to the autophagic membrane-related protein LC3 during the process of mitochondrial depolarization and proteasome-dependent outer membrane rupture, which is closely associated with the PINK1/Parkin signaling pathway. (6) Cardiolipin (CL), as a phospholipid in the inner mitochondrial membrane, can be externalized to the outer mitochondrial membrane when mitochondria are damaged, while the redistribution of CL and its interaction with LC3 initiate a signaling cascade and mediate mitochondrial autophagy.