Abstract
For prognostic assessment in women who receive radiotherapy (RT) for bone metastases (BMs) from breast cancer (BC), prognostic factors specific for BMs from BC were investigated in the present study. The prognostic assessment was performed by retrospectively reviewing 143 women who received first-time RT for BMs from BC between January 2007 and June 2018. The median follow-up time and median overall survival (OS) time from the first-time RT for BMs were 22 and 18 months, respectively. In the multivariate analysis, nuclear grade 3 (NG 3) [hazard ratio, 2.18; 95% confidence interval (CI), 1.34-3.53], brain metastases (hazard ratio, 1.96; 95% CI, 1.01-3.81), liver metastases (hazard ratio, 1.75; 95% CI, 1.17-2.63), performance status (PS) (hazard ratio, 1.63; 95% CI, 1.10-2.41) and previous systemic therapy (hazard ratio, 1.58; 95% CI, 1.03-2.42) were significant factors for OS, whereas age, hormone-receptor/human epidermal growth factor receptor 2 status, number of BMs and synchronous lung metastases were not significant factors. When points according to risk levels [unfavorable points (UFPs)] were assigned to each risk factor (1.5 points for NG 3 and brain metastases; and 1 point for PS ≥2, previous systemic therapy and liver metastases), the median OS times of patients with a total number of UFPs ≤1 (n=45), 1.5–3 (n=55) and ≥3.5 (n=43) were 36, 17 and 6 months, respectively. Overall, in patients who received first-time RT for BMs from BC, NG 3, brain/liver metastases, poor PS and previous systemic therapy were unfavorable prognostic factors. Comprehensive prognostic assessment using these factors seemed to be useful for the prediction of prognoses in patients with BMs from BC.
Keywords: bone metastases, breast neoplasm, individualized medicine, prognosis, radiotherapy
Introduction
The bones are the primary metastatic site in 46% of patients with breast cancer (BC), and bone metastases (BMs) have been reported to occur in 71% of metastatic BC cases (1). In patients with BC and BMs, although the main treatment strategy is systemic therapy, palliative radiotherapy (RT) for BMs is often used for pain relief. In general, the majority of patients who need palliative RT for BMs cannot expect long-term survival. Therefore, hypofractionated low-dose RT (e.g. a single fraction of 8 Gy) for pain relief is often performed for BMs. However, the prognosis of patients with BMs is heterogeneous, and the prognosis of patients with BMs from BC is considered to be favorable (2,3). Therefore, to select the appropriate RT methods for BMs from BC, precise prediction of life expectancy is crucial in patients with BC and BMs.
Svensson et al (2) reported that the 1-year survival rate after the diagnosis of BMs was lowest in patients with lung cancer (10%) and highest in patients with BC (51%), and that 1 in 10 patients with BMs from BC survived for 5 years. In Katagiri's prognostic scoring system for patients with BMs from various cancer types, BC was classified as a comparatively favorable cancer type (3). In addition, BC has individual features; unlike other cancer types, the prognoses of patients with BC are affected by positive hormone receptor expression, human epidermal growth factor receptor 2 (HER2) overexpression and histological grade (4,5). Furthermore, the majority of patients with BC who receive RT for BMs have already received systemic therapy, including chemotherapy and/or hormonal therapy. After systemic therapy, there is a possibility that the features of the BC cells have changed from those at the initial presentation (6). Although generalized prognostic assessment systems for patients with BM, such as the Katagiri score (3) and Tokuhashi score (7), have been proposed, there are few prognostic assessment systems that are specialized for patients with BMs from BC. Considering the high incidence of BMs from BC and the individual features of BC, prognostic assessments specific to BMs from BC are needed. For individualized RT, prognostic factors specific to patients who received RT for BMs from BC were therefore assessed in the present study.
Materials and methods
Ethical considerations
All procedures performed in the present study were in accordance with the ethical standards of the Institutional and/or National Research Committee and the 1964 Helsinki Declaration and its later amendments. Informed consent for the use of clinical data was obtained by opt-out methods. This retrospective study was approved by the Ethics Committee of the National Hospital Organization Shikoku Cancer Center (Matsuyama, Japan; approval no. RIN2021-71).
Study population
The cases of 167 consecutive female patients who received first-time RT for BMs from BC between January 2007 and June 2018 in the National Hospital Organization Shikoku Cancer Center were retrospectively reviewed. A total of 24 patients were excluded for the following reasons: i) No computed tomography (CT) of the chest and abdomen within 3 months of beginning RT (n=14); ii) discontinuation of RT (n=2); iii) synchronous and/or sequential double cancer (n=5); and iv) follow-up duration of <6 months despite survival (n=3). Thus, 143 patients were included for analysis. Among the 143 patients, 117 patients (82%) had died and 26 patients (18%) were alive at the last follow-up day of clinical examination.
BMs were detected with CT, bone scintigraphy and/or 18F fluorodeoxyglucose positron-emission tomography/CT (FDG-PET/CT). Visceral metastases were evaluated with CT and/or FDG-PET/CT within 3 months of the beginning of first-time RT to BMs. Patients who had not undergone contrast-enhanced magnetic resonance imaging (MRI) were classified in a no brain metastases cohort. Performance status (PS) was evaluated according to the Eastern Cooperative Oncology Group scale (8).
Radiotherapy
Patients received three-dimensional conformal RT. RT was delivered using 4- or 10-MV photons with a linear accelerator (Clinac 21-EX; Varian Medical Systems, Inc.).
Statistical analysis
The primary endpoint was overall survival (OS) time, which was defined as the time from the beginning of the first-time RT for BMs to death. OS was calculated using the Kaplan-Meier method, and statistical differences in OS were evaluated by the log-rank test. The Cox proportional hazard model was used for univariate and multivariate analyses. Factors, including age, PS, estrogen receptor status, progesterone receptor status, HER2 overexpression, triple-negative BC type, nuclear grade (NG) (9), the timing of the appearance of metastases (relapse vs. de novo), previous systemic therapy, number of BMs (single vs. multiple) and sites of synchronous metastases (lung, liver and brain), were analyzed using univariate analysis. Factors that had P-values of <0.05 on univariate analysis were subjected to multivariate analysis. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing; version 3.5.0) (10). More precisely, it is a modified version of R commander (2.5-1), designed to incorporate statistical functions frequently used in biostatistics.
Results
Clinical characteristics
A total of 128 patients (90%) had multiple BMs (only vertebral, 7 patients; only non-vertebral, 5 patients; both vertebral and non-vertebral, 116 patients) and 15 patients (10%) had a single bone metastasis (vertebral, 11 patients; non-vertebral, 4 patients). Overall, 98 patients (69%) had undergone previous systemic therapy, including chemotherapy, hormone therapy and/or anti-HER2 agent therapy for >3 months before RT. A total of 58 patients (41%) underwent FDG-PET/CT before the RT. In addition, 39 patients (27%) underwent contrast-enhanced T1-weighted MRI (CE-MRI) within 3 months of the beginning of the RT or during RT, and brain metastases were detected in 14 patients (10%).
The majority of patients received 30 Gy in 10 fractions. Overall, 130 patients (91%) received at least 30 Gy in 10 fractions and 13 patients (9%) received hypofractionated low-dose RT. A total of 108 patients (76%) received RT to the vertebral bone and 29 patients (20%) received RT to the pelvic bone. Patient characteristics are listed in Table I.
Table I.
Patient characteristics.
| Characteristic | Value |
|---|---|
| Median age (range), years | 61 (33–88) |
| PS, n (%) | |
| 0 | 9 (6.3) |
| 1 | 66 (46.2) |
| 2 | 35 (24.5) |
| 3 | 19 (13.3) |
| 4 | 12 (8.4) |
| Unknown | 2 (1.4) |
| Timing of appearance of metastases, n (%) | |
| Relapse | 97 (67.8) |
| de novo | 46 (32.2) |
| ER, n (%) | |
| Positive | 117 (81.8) |
| Negative | 19 (13.3) |
| Unknown | 7 (4.9) |
| PgR, n (%) | |
| Positive | 95 (66.4) |
| Negative | 41 (28.7) |
| Unknown | 7 (4.9) |
| HER2 overexpression, n (%) | |
| Yes | 20 (14.0) |
| No | 110 (76.9) |
| Unknown | 13 (9.1) |
| TN type, n (%) | |
| Yes | 9 (6.3) |
| No | 124 (86.7) |
| Unknown | 10 (7.0) |
| NG, n (%) | |
| 1 | 20 (14.0) |
| 2 | 24 (16.8) |
| 3 | 56 (39.2) |
| Unknown | 43 (30.1) |
| Previous systemic therapy, n (%) | |
| Yes | 98 (68.5) |
| No | 45 (31.5) |
| Number of bone metastases, n (%) | |
| Single | 15 (10.5) |
| Multiple | 128 (89.5) |
| RT sites, n (%) | |
| Vertebral | 108 (75.5) |
| Pelvis | 29 (20.3) |
| Other | 6 (4.2) |
| Brain metastases | |
| Yes | 14 (9.8) |
| No | 25 (17.5) |
| Not examined | 104 (72.8) |
| Liver metastases | |
| Yes | 55 (38.5) |
| No | 88 (61.5) |
| Lung metastases | |
| Yes | 56 (39.2) |
| No | 87 (60.8) |
PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TN, triple-negative; NG, nuclear grade; RT, radiotherapy.
The median follow-up duration of survival was 22 months (range, 7–117 months) from first-time RT for BMs. The median OS time was 17.5 months. The OS curve is shown in Fig. 1.
Figure 1.
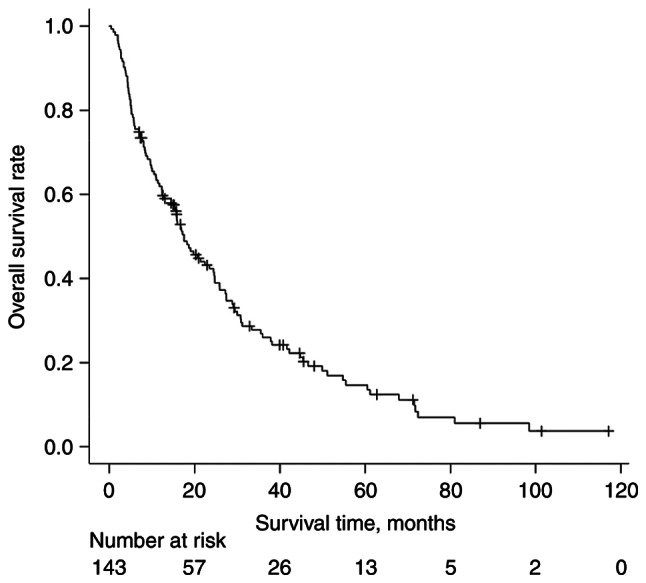
Kaplan-Meier curve of overall survival rate in patients who received radiotherapy for bone metastases from breast cancer.
Prognostic factors for patients who received first-time RT for BMs from BC
Upon univariate analysis, PS ≥2 (P=0.021), NG 3 (P<0.001), previous systemic therapy (P=0.003), synchronous brain metastases (P<0.001), synchronous liver metastases (P<0.001) and synchronous lung metastases (P=0.006) were significantly associated with poor OS after first-time RT for BMs (Table II). There were no significant differences in OS between PS 0–1 and unknown PS (P=0.260), and between NG 1–2 and unknown NG (P=0.956).
Table II.
Univariate analysis for overall survival.
| Characteristic | 1-year OS rate, % | P-value | HR (95% CI) |
|---|---|---|---|
| Age, years (<60 vs. ≥61) | 62.4 vs. 61.4 | 0.943 | 1.01 (0.70-1.46) |
| PS (0, 1 vs. ≥2) | 76.8 vs. 45.5 | 0.021 | 1.55 (1.07-2.24) |
| Timing of appearance of metastases (relapse vs. de novo) | 59.2 vs. 67.4 | 0.182 | 0.77 (0.52-1.13) |
| ER (positive vs. negative) | 63.8 vs. 53.1 | 0.141 | 1.49 (0.88-2.55) |
| PgR (positive vs. negative) | 62.0 vs. 61.9 | 0.630 | 1.11 (0.73-1.68) |
| HER2 overexpression (positive vs. negative) | 65.0 vs. 61.4 | 0.441 | 1.24 (0.72-2.15) |
| TN type (yes vs. no) | 40.0 vs. 63.6 | 0.311 | 0.68 (0.33-1.40) |
| NG (1, 2 vs. 3) | 74.9 vs. 43.5 | <0.001 | 2.30 (1.46-3.60) |
| Previous systemic therapy (no vs. yes) | 73.3 vs. 56.6 | 0.003 | 1.84 (1.22-2.76) |
| Number of bone metastases (single vs. multiple | 60.0 vs. 62.1 | 0.774 | 1.10 (0.59-2.05) |
| RT sites (vertebral vs. non-vertebral) | 64.6 vs. 53.3 | 0.093 | 1.42 (0.94-2.14) |
| Brain metastases (no vs. yes) | 65.8 vs. 23.1 | <0.001 | 3.06 (1.67-5.62) |
| Liver metastases (no vs. yes) | 71.4 vs. 46.5 | <0.001 | 2.20 (1.51-3.20) |
| Lung metastases (no vs. yes) | 70.1 vs. 48.4 | 0.006 | 1.73 (1.17-2.54) |
OS, overall survival; HR, hazard ratio; CI, confidence interval; PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TN, triple-negative; NG, nuclear grade; RT, radiotherapy.
The 1-year OS rate was as follows: PS 0–1 vs. PS 2–4, 76.8 vs. 45.5%; NG 1–2 vs. NG 3, 74.9 vs. 43.5%; previous chemotherapy yes vs. no, 56.6 vs. 73.3%; synchronous brain metastases yes vs. no, 23.1 vs. 65.8%; synchronous liver metastases yes vs. no, 46.5 vs. 71.4%; and synchronous lung metastases yes vs. no, 48.4 vs. 70.1% (Table II).
Upon multivariate analysis, the significant prognostic factors after first-time RT for BMs were NG 3 [hazard ratio, 2.18; 95% confidence interval (CI), 1.34-3.53; P=0.002], synchronous brain metastases (hazard ratio, 1.96; 95% CI, 1.01-3.81; P=0.046), synchronous liver metastases (hazard ratio, 1.75; 95% CI, 1.17-2.63; P=0.006), PS (hazard ratio, 1.63; 95% CI, 1.10-2.41; P=0.016) and previous systemic therapy (hazard ratio, 1.58; 95% CI, 1.03-2.42; P=0.038). Age, the timing of the appearance of metastases, hormone-receptor status, HER2 status, number of bone metastases (single vs. multiple) and synchronous lung metastases were not significant factors.
Comprehensive prognostic assessment based on regression coefficients in patients who received first-time RT for BMs from BC
A comprehensive prognostic assessment using regression coefficients of significant prognostic factors in multivariate analysis (Table III) was performed. Points according to risk levels of each significant prognostic factor [unfavorable points (UFPs)] were assigned to each prognostic factor as follows: 1.5 points for NG 3 and synchronous brain metastases, and 1 point for PS ≥2, previous systemic therapy and synchronous liver metastases (Table IV). Patients with BMs from BC were classified into three groups stratified by their total UFPs as follows: i) Favorable group with total UFPs of ≤1 (n=45); ii) intermediate group with total UFPs of 1.5–3 (n=55); and iii) unfavorable group with total UFPs of ≥3.5 (n=43). The median OS time was 36 months for the favorable group, 17 months for the intermediate group and 6 months for the unfavorable group. There were statistically significant differences in OS time between the three groups (P<0.001). The OS curves are shown in Fig. 2.
Table III.
Multivariate analysis for overall survival.
| Characteristic | Regression coefficient | P-value | HR (95% CI) |
|---|---|---|---|
| PS (≥2 vs. 0–1) | 0.4860 | 0.016 | 1.63 (1.10–2.41) |
| NG (3 vs. 1–2) | 0.7776 | 0.002 | 2.18 (1.34–3.53) |
| Previous systemic therapy (yes vs. no) | 0.4543 | 0.038 | 1.58 (1.03–2.42) |
| Brain metastases (yes vs. no) | 0.6747 | 0.046 | 1.96 (1.01–3.81) |
| Liver metastases (yes vs. no) | 0.5621 | 0.006 | 1.75 (1.17–2.63) |
| Lung metastases (yes vs. no) | 0.1131 | 0.627 | 1.12 (0.71–1.77) |
HR, hazard ratio; CI, confidence interval; PS, Eastern Cooperative Oncology Group performance status; NG, nuclear grade.
Table IV.
UFPs for significant prognostic factors.
| Factor | UFPs |
|---|---|
| PS | |
| ≥2 | 1 |
| 0–1 | 0 |
| NG | |
| 3 | 1.5 |
| 1–2 | 0 |
| Previous systemic therapy | |
| Yes | 1 |
| No | 0 |
| Brain metastases | |
| Yes | 1.5 |
| No | 0 |
| Liver metastases | |
| Yes | 1 |
| No | 0 |
UFP, unfavorable point; PS, performance status; NG, nuclear grade.
Figure 2.
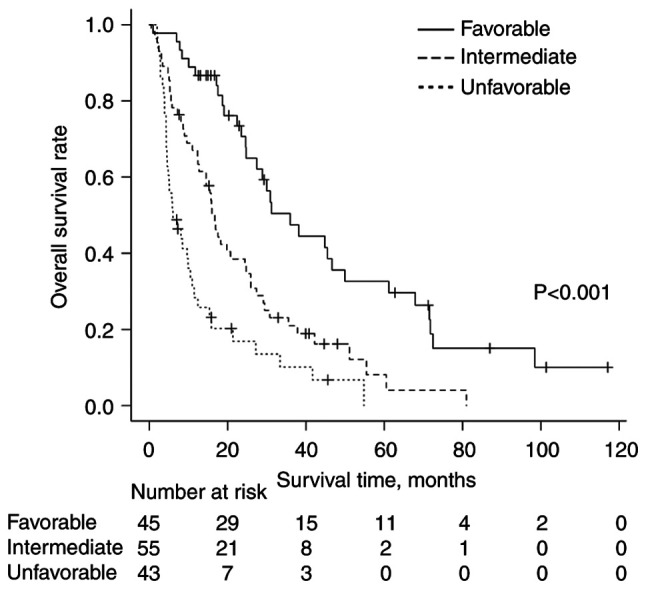
Kaplan-Meier curves of overall survival rates according to total UFPs in patients who received radiotherapy for bone metastases from breast cancer. The favorable group (total UFPs ≤1), the intermediate group (total UFPs=1.5-3) and the unfavorable group (total UFPs ≥3.5) are shown by the solid, dashed, and dotted lines, respectively. UFPs, unfavorable points.
Discussion
For individualization of RT to BMs from BC, prognostic factors for patients who received first-time RT to BMs from BC were investigated in the present study. Based on the multivariate analysis, NG 3, synchronous brain metastases, synchronous liver metastases, PS ≥2 and previous systemic therapy were the statistically significant unfavorable prognostic factors for OS in patients who received first-time RT for BMs from BC. By contrast, hormone receptor status, HER2 status, the timing of appearance of metastases (relapse vs. de novo), number of BMs (single vs. multiple) and synchronous lung metastases were not significant factors. In addition, a comprehensive prognostic assessment using total UFPs according to risk levels of each unfavorable factor seemed to be useful to predict OS after first-time RT for BMs. OS was significantly poorer in patients whose total UFPs were higher.
In clinical practice, RT with variable dose-fractionation schedules (e.g. single fraction RT of 8 Gy, traditional RT with 30 Gy in 10 fractions and stereotactic body RT) is administered to BMs. According to a meta-analysis, single-fraction RT was not inferior to multiple-fraction RT regarding pain relief from BMs (11). However, re-irradiation was more frequent for single-fraction RT (11). Differences in local control according to dose-fractionation schedules have been reported in several studies regarding metastatic spinal cord compression (MSCC) (12,13). Rades et al (12) reported that RT of 30 Gy in ≥10 fractions was superior to 20 Gy in ≤5 fractions in local control for all patients with MSCC (median OS time of assessed patients was 13 months). However, the same group also found that there were no significant differences in local progression-free survival time between 30 Gy in 10 fractions and 20 Gy in 5 fractions when limited to patients with poor or intermediate prognosis (median OS time of assessed patients was 3.2 months) (13). Considering these results, hypofractionated low-dose RT is likely to be inadequate for long-term survivors. Highly precise RT using stereotactic body RT or intensity-modulated RT can now be performed if necessary. The prediction of prognosis is more important for BMs from BC compared with BMs from other primary sites.
Histological grade or NG has been one of the most often used major prognostic factors for operable BC, along with lymph node metastases and tumor size (14,15). From the results of the present study, NG 3 was an independent unfavorable prognostic factor also for patients with BMs from BC.
Synchronous metastases in other organs also affect the prognoses of patients with BMs from BC. According to previous reports regarding patients with BMs from BC, patients who have synchronous metastases in other organs showed shorter median OS times (median OS time, 9–17 months) compared with patients with bone-only metastases (median OS time, 31–33 months) (4,5). Chen et al (4) reported that brain, liver and lung metastases were significantly associated with poor OS in patients with metastatic BC. In addition, prognoses of patients with brain metastases and patients with multiple site metastases had the poorest prognosis. Largillier et al (5) reported that brain metastases and liver metastases were significant unfavorable factors for survival in metastatic BC, regardless of hormone receptor status. In addition, Gerratana et al (16) reported that liver metastasis was an independent predictor for poor OS in patients who had received anticancer therapy for metastatic BC. From the results of these studies, brain metastases and liver metastases seemed to be especially unfavorable factors in patients with metastatic BC. In the present study, regarding patients who had BMs and synchronous metastases at other organs, the prognosis was unfavorable in the order of brain, liver and then lung metastasis.
Based on the results from multivariate analysis, previous systemic therapy was also a significantly unfavorable prognostic factor in the present study. Previous systemic therapy has the potential to induce resistance to systemic therapy and is likely to lead to an unfavorable prognosis.
In the present study, hormone-receptor status and HER2 status were not significant factors. There was a possibility that the hormone-receptor status and HER2 status at the initial presentation had changed after previous systemic therapy. Features of tumor cells in metastatic lesions sometimes differ from those in primary lesions (17). Approximately 70% of the present study patients had received previous systemic therapy. This may be one of the possible explanations that the hormone-receptor/HER2 status and TN type were not significant factors for OS in patients who received first-time RT to BMs in the study. Furthermore, the number of BMs (single vs. multiple) also was not a significant factor. Previous systemic therapy may also contribute to this result.
Comprehensive prognostic assessment using the designated UFPs seemed to be useful for the prediction of the prognoses of patients with BMs from BC. The median survival time of the favorable group (total UFPs ≤1) was >3 years. Well-fractionated higher-dose RTs are likely to be suitable for these patients. By contrast, single fraction RT of 8 Gy is suitable for the unfavorable group (total UFPs ≥3.5). Prognostic assessment using total UFPs was helpful to determine the appropriate dose-fractionation schedules for patients with BMs from BC.
The present study has several limitations, including its retrospective nature. First, due to the lack of laboratory data in numerous cases, the prognostic assessment using UFPs cannot be compared to Katagiri's prognostic scoring system (3), which is very detailed. However, clinically, a number of cases do not have the laboratory data used in Katagiri's prognostic scoring system. The present prognostic assessment using UFPs does not require these data and can therefore be used in such cases. Second, the quality of life of patients with BC and BMs after palliative RT could not be assessed, which is an important factor in palliative treatment; moreover, the medical records did not provide sufficient data for evaluating these factors. Third, the sample size was small and insufficient to validate the prognostic scoring system more precisely. Therefore, large-scale prospective studies are required in the future for validating this prognostic system.
In conclusion, PS ≥2, NG 3, previous systemic therapy, synchronous brain metastases and synchronous liver metastases were significantly associated with poor OS time for patients with BMs from BC. A comprehensive prognostic assessment using UFPs based on these factors seemed to be useful to select patients who needed comparatively well-fractionated high-dose RT for BMs from BC.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
KM, HK and YH designed the study concepts. KM, HK, YH, KN, MK, SO and TK collected patient data, and analyzed and interpreted the data. KM, HK and YH confirm the authenticity of all the raw data. KM, HK, YH, KN, MK, SO and TK collaborated in the discussion. KM, HK and YH prepared the manuscript, and KN, MK, SH and TK revised it critically for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the National Hospital Organization Shikoku Cancer Center (Matsuyama, Japan; approval no. RIN2021-71). The opt-out method was applied with regard to consent for this study.
Patient consent for publication
Not applicable.
Competing interests
SO received honorarium from AstraZeneca plc. All other authors declare that they have no competing interests.
References
- 1.Solomayer EF, Diel IJ, Meyberg GC, Gollan C, Bastert G. Metastatic breast cancer: Clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat. 2000;59:271–278. doi: 10.1023/A:1006308619659. [DOI] [PubMed] [Google Scholar]
- 2.Svensson E, Christiansen CF, Ulrichsen SP, Rorth MR, Sorensen HT. Survival after bone metastasis by primary cancer type: A Danish population-based cohort study. BMJ Open. 2017;7:e016022. doi: 10.1136/bmjopen-2017-016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katagiri H, Okada R, Takagi T, Takahashi M, Murata H, Harada H, Nishimura T, Asakura H, Ogawa H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3:1359–1367. doi: 10.1002/cam4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen MT, Sun HF, Zhao Y, Fu WY, Yang LP, Gao SP, Li LD, Jiang HL, Jin W. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: A SEER population-based analysis. Sci Rep. 2017;7:9254. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F, Birtwisle-Peyrottes I, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19:2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 7.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 8.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Black MM, Barclay TH, Hankey BF. Prognosis in breast cancer utilizing histologic characteristics of the primary tumor. Cancer. 1975;36:2048–2055. doi: 10.1002/cncr.2820360919. [DOI] [PubMed] [Google Scholar]
- 10.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow R, Hoskin P, Schild SE, Raman S, Im J, Zhang D, Chan S, Chiu N, Chiu L, Lam H, et al. Single vs multiple fraction palliative radiation therapy for bone metastases: Cumulative meta-analysis. Radiother Oncol. 2019;141:56–61. doi: 10.1016/j.radonc.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Rades D, Lange M, Veninga T, Stalpers LJ, Bajrovic A, Adamietz IA, Rudat V, Schild SE. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79:524–530. doi: 10.1016/j.ijrobp.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 13.Rades D, Segedin B, Conde-Moreno AJ, Garcia R, Perpar A, Metz M, Badakhshi H, Schreiber A, Nitsche M, Hipp P, et al. Radiotherapy with 4 Gy × 5 versus 3 Gy × 10 for metastatic epidural spinal cord compression: Final results of the SCORE-2 trial (ARO 2009/01) J Clin Oncol. 2016;34:597–602. doi: 10.1200/JCO.2015.64.0862. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Mori I, Sakurai T, Yoshimura G, Suzuma T, Nakamura Y, Nakamura M, Taniguchi E, Tamaki T, Umemura T, Kakudo K. Correlation between nuclear grade and biological prognostic variables in invasive breast cancer. Breast Cancer. 2001;8:105–110. doi: 10.1007/BF02967488. [DOI] [PubMed] [Google Scholar]
- 15.Rakha EA, El-Sayed ME, Lee AH, Elston CW, Grainge MJ, Hodi Z, Blamey RW, Ellis IO. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26:3153–3158. doi: 10.1200/JCO.2007.15.5986. [DOI] [PubMed] [Google Scholar]
- 16.Gerratana L, Fanotto V, Bonotto M, Bolzonello S, Minisini AM, Fasola G, Puglisi F. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis. 2015;32:125–133. doi: 10.1007/s10585-015-9697-2. [DOI] [PubMed] [Google Scholar]
- 17.Curigliano G, Bagnardi V, Viale G, Fumagalli L, Rotmensz N, Aurilio G, Locatelli M, Pruneri G, Giudici S, Bellomi M, et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann Oncol. 2011;22:2227–2233. doi: 10.1093/annonc/mdq751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


