Figure 1. Patient Screening, Randomization, and Participation in the TXA-127 Trial and in the TRV-027 Trial.
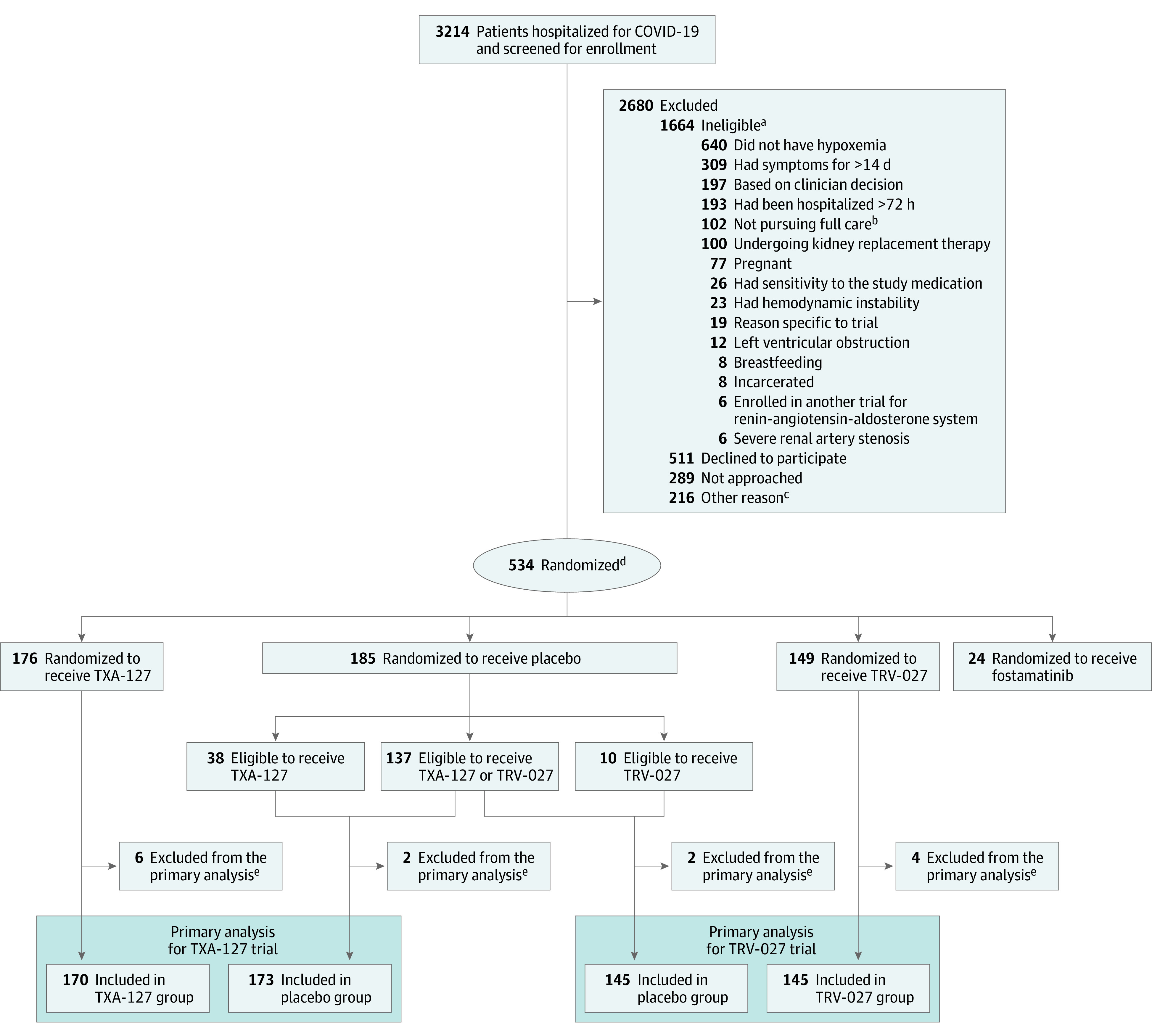
aThe criteria were not mutually exclusive; some potential participants met multiple criteria for ineligibility.
bThe patient, clinical team, or both, was not pursuing full medical management (eg, a do not intubate order).
cIncluded (1) the patient was to be discharged from the hospital before the study procedures could be initiated, (2) the patient was enrolled in another trial, and (3) the patient’s situation presented logistical challenges for trial enrollment.
dPatients eligible for more than 1 trial were randomized with equal probability to a specific trial. Patients were randomized to active vs placebo in an m:1 ratio, with m representing the number of trials for which the patient was eligible. Patients randomized to placebo were included in the placebo groups of each trial for which they were eligible. Patients randomized to receive an active drug contributed only to the trial they were randomized. Thus, patients randomized to receive active fostamatinib did not contribute to either the TXA-127 trial or the TRV-027 trial, whereas patients assigned to placebo fostamatinib were included in the placebo groups for the TXA-127 and TRV-027 trials if they were eligible for those trials. This design with a shared placebo group reduces the total number of patients who received placebo while retaining a statistically efficient 1:1 active vs placebo allocation for each trial.
eDid not receive the randomized study drug or were randomized but did not meet the enrollment criteria.
