Abstract
Meningitis and encephalitis are neurologic emergencies that require immediate management and current guidelines recommend empiric treatment with broad-spectrum antimicrobials. Cerebrospinal fluid (CSF) testing algorithms are heterogeneous and largely institution-specific, reflecting a lack of consensus on how to effectively identify CSF pathogens while conserving resources and avoiding false positives. Moreover, many lumbar punctures (LPs) performed in the inpatient setting are done for noninfectious workups, such as evaluation for leptomeningeal metastasis. As such, tailoring CSF testing to clinical context has been a focus of multiple prior reports and several healthcare systems have focused on efforts to limit low-yield diagnostic testing when a positive result is unlikely. To curb ordering viral PCRs when pre-test probability is low, some peer institutions have implemented pleocytosis criteria for virus-specific polymerase chain reaction (PCR) tests from CSF. In this report, we retrospectively analyzed the diagnostic testing of CSF from patients who had an LP while admitted to a single, large academic medical center and found that many cases of Herpes Simplex Virus (HSV) meningoencephalitis were diagnosed by non-neurologists. The rate of positive virus-specific PCR tests was very low, and tests were frequently ordered in duplicate with a multiplexed meningitis/encephalitis PCR panel (M/E panel, BioFire, Salt Lake City, UT). We designed and implemented a systems-level intervention to promote a revised stepwise testing algorithm that minimizes unnecessary tests. This intervention led to a significant reduction in the number of low-yield virus-specific PCR tests ordered without implementing a policy of cancelling virus-specific PCRs.
Keywords: meningoencephalitis < central nervous system infections, quality < techniques, biostatistics < techniques, neurocritical care < clinical specialty, neurohospitalist < clinical specialty
Introduction
CSF testing is essential for the workup of meningitis and encephalitis, although data suggests that that many virus-specific PCRs are ordered despite a low pre-test probability of a positive result.1-3 Unnecessary testing exposes patients to the risk of false positives and increases the cost of care, which makes diagnostic stewardship an important focus for quality improvement (QI) efforts. 4 The electronic health record (EHR) may inadvertently promote low-yield diagnostic testing with a la carte choices, 5 and one group noted that introducing an EHR led to a significant increase in virus-specific PCR tests ordered from CSF. 3 Conversely, optimization of the EHR may provide focused decision support and limit unnecessary testing,6,7 which justifies an emphasis on EHR ‘order sets’ for efforts designed to improve diagnostic stewardship. 8 ‘Order sets’ are menus of lab tests for common clinical scenarios in the EHR that allow users to select from a prespecified list of tests instead of requiring users to recall each test and search for it from a database.
Increasing testing volumes, especially in contexts with a low pre-test probability, has led several institutions to use pleocytosis as a necessary condition for virus-specific PCR testing from CSF.1-3 At our own institution, we noted a high rate of virus-specific PCR testing from CSF (below), but sought an alternative solution to a pleocytosis-based criteria given the large immunocompromised population at our center. Virus-specific PCR tests are often ordered twice on the same specimen: once in a syndromic meningitis/encephalitis PCR panel (M/E panel, BioFire, Salt Lake City, UT), and again using a dedicated virus-specific PCR. 9 The M/E panel can potentially result within a few hours after sample collection, whereas virus-specific PCRs are batched and require days to result. The rapid turnaround time of the M/E panel may occur at the cost of sensitivity, with meta-analyses describing sensitivities of 75.5-93.8% for HSV1, HSV2, and varicella zoster (VZV).10,11 However, we have previously noted that the positive predictive value of the M/E panel for HSV and VZV is very high. 9
Given the differences between the M/E panel and virus-specific PCR tests, we designed a CSF testing algorithm intended to limit the breadth of initial CSF testing. As part of this algorithm, the CSF specimen is held so that additional focused tests may be subsequently ordered, guided by initial test results and clinical suspicion. A similar paradigm for CSF testing was previously proposed, 4 but did not incorporate the M/E panel. Here we characterize historic ordering practices for CSF virus-specific PCRs and describe our efforts to limit duplicative and unnecessary testing.
Methods
Ethics
Per our Institutional Review Board (IRB), this project constituted a quality improvement project and was exempt from IRB approval.
Study Design
We performed 3 key analyses using adult inpatient data. For Analysis 1, we performed a retrospective cohort study of inpatients who underwent a lumbar puncture (LP) with diagnostic tests ordered from the inpatient LP orderset from 6/14/2021-9/16/2021, a three-month convenience sample at the beginning of the project. Data collected included the service performing the LP and the number of CSF tests ordered per LP. We defined neurology providers as neurology residents, fellows, and neuro-ICU advanced practice providers (APPs).
In Analysis 2, we completed a retrospective chart review using a database of all positive M/E panel results spanning 6/2017-9/2021. This analysis included service performing the LP and the M/E panel result.
For Analysis 3, we prospectively monitored the effect of our intervention on ordering practices using Epic (Epic Systems, Verona, WI) informatics tools. Historic data from 1/1/2019 onwards is included for context and data from 3/1/2022 onwards was collected prospectively (Figure 2). This data included the test result (positive/negative) and the other diagnostic tests ordered contemporaneously.
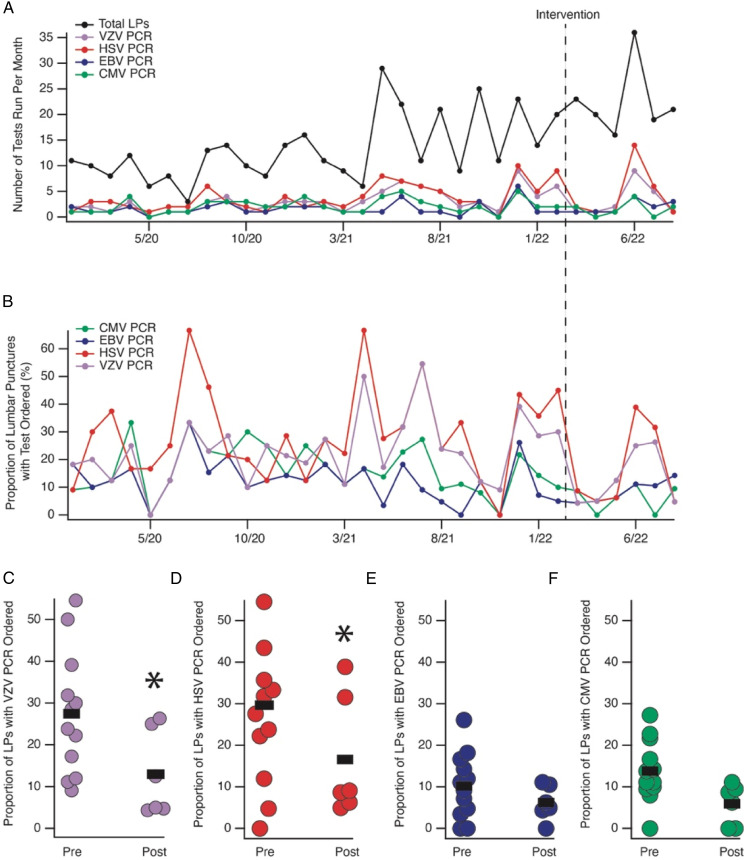
Figure 2.
Raw number of LPs and virus-specific PCRs performed with the inpatient LP order set on a month-to-month basis (A) and scaled as a function of number of LPs (B). Month of inpatient LP revision shown with a vertical dashed line marked ‘Intervention’. (C-F) Plots of the proportion of LPs performed with the test in question ordered. Each month is represented by a dot such that ‘50%’ denotes that half of the LPs performed in that month had the quest ordered. Note that 12 months pre-intervention and 6 months post-intervention are shown with a black line denoting the mean. *denotes P < .05 by Chi-Square testing, see Table 1 for comparisons.
Intervention
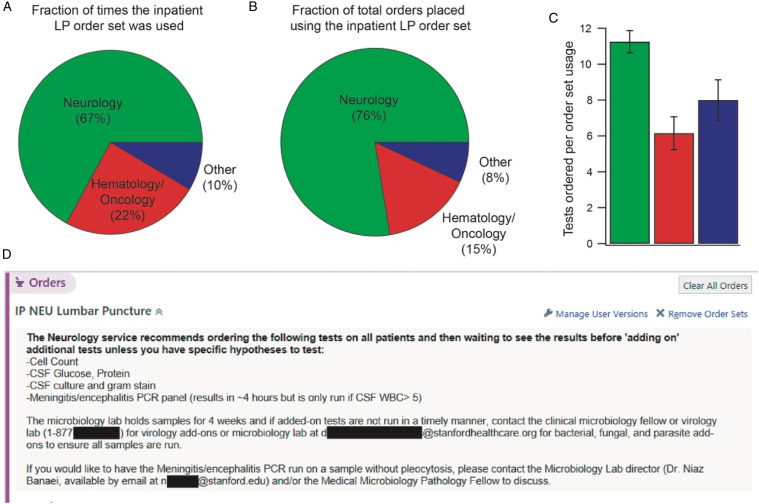
We revised the inpatient LP order set to include specific recommendations intended to reduce the breadth of initial CSF testing and encourage subsequent focused testing based on initial results and clinical suspicion (Figure 1D). To educate Neurology trainees about the recommended workflow for ordering CSF tests, a department-wide ‘Grand Rounds’ presentation was given in March 2022 by one author (KL).
Figure 1.
A) Usage of the inpatient LP order set by each service represented as a pie chart during a 3-month span. B) Fraction of the total orders placed in the same 3-month span that were placed by each service. C) Number of tests ordered per usage of the inpatient LP order set by each service. D) Graphic showing the language introduced to the inpatient LP order set that constituted our intervention and which was visible by all users of the inpatient LP order set.
Statistical Analyses
The chi-square test was used to compare differences in ordering rates. All statistical tests were computed for a two-sided type I error rate of 5%. The difference in observed and expected tests for each of the 3 significantly different tests (HSV, VZV, CMV) was extrapolated over one year to estimate total tests avoided.
Results
Analysis 1: Of the 82 LPs documented from 6/14/2021-9/16/2021 and performed using the inpatient LP order set, 67% were completed by neurology providers (Figure 1A), 25% by hematology/oncology providers, and 9% were ‘other’ (medicine residents, anesthesia residents, and medical ICU APPs). There were no emergency department or neurosurgery providers, indicating they do not use the inpatient LP order set. In addition to performing the most LPs using this order set, neurology providers ordered the most tests per LP (Figure 1B).
We next examined the number of virus-specific PCRs ordered from the inpatient LP order set (Figure 2A) and the rate at which these virus-specific PCRs returned positive. The HSV PCR was positive 2% of the time (2/125), the VZV PCR was positive 0% of the time (0/108), the CMV PCR was positive 0% of the time (0/63), and the EBV PCR was positive 6% of the time (3/53), similar to other reports.1-3
Analysis 2: In our prior report, we observed more cases of HSV encephalitis than were being captured in our current analysis. 9 We reviewed positive M/E PCR panels and identified 14 cases of HSV encephalitis diagnosed using the M/E PCR. Given that we observed just 2/125 HSV PCRs that were positive, we investigated these 14 cases identified with the M/E panel further by reviewing the EHR for each case. Just 2 of the 14 cases were diagnosed on tests ordered by neurology team members (who often use the inpatient LP order set) whereas the remaining cases were diagnosed by other services (8 by emergency department providers, 3 by medical ICU providers, and 1 by an internal medicine provider). In 7 of the 14 cases, the neurology service was involved in the patient’s care prior to LP, either as a consulting service or the primary admitting team. In the remaining 7 cases the LP was completed prior to neurology being consulted (2 cases) or the patient was managed without neurology input (5 cases). In 13 of the 14 cases, the HSV PCR was ordered on the same CSF specimen. In 8 cases it was ordered simultaneous with the M/E PCR and in 5 cases it was ordered afterwards, presumably to ‘confirm’ the M/E PCR result.
Analysis 3: Based on the results outlined above, we added specific recommendations to the inpatient LP order set (Figure 1D) and educated the neurology providers on the rationale for this approach in a department-wide presentation. Six months after our intervention, we found a statistically significant reduction in the frequency of HSV, VZV, and CMV PCRs ordered (Table 1). Extrapolated over 1 year, this translates into 86 fewer virus-specific PCRs ordered.
Table 1.
2 × 2 tables highlighting the number of times each virus-specific PCR was ordered in the 12-month period prior to the intervention in comparison with the 6-month period after the intervention. The results of Chi square testing are shown to the right for each comparison.
| VZV | LPs with Test Ordered | LPs without Test Ordered | ||
|---|---|---|---|---|
| Pre-Intervention | 52 | 148 | X2 | P value |
| Post-Intervention | 19 | 116 | 6.863 | .008 |
| HSV | LPs with test ordered | LPs without test ordered | ||
| Pre-Intervention | 62 | 138 | X2 | P value |
| Post-Intervention | 25 | 110 | 6.530 | .010 |
| EBV | LPs with test ordered | LPs without test ordered | ||
| Pre-Intervention | 20 | 180 | X2 | P value |
| Post-Intervention | 12 | 123 | .115 | .734 |
| CMV | LPs with test ordered | LPs without test ordered | ||
| Pre-Intervention | 28 | 172 | X2 | P value |
| Post-Intervention | 9 | 126 | 4.411 | .035 |
Discussion
In this QI project, we aimed to foster diagnostic stewardship by reducing the number of duplicative and unnecessary virus-specific PCRs ordered on CSF without implementing a pleocytosis-based cutoff.8,12 Our EHR-based intervention changed ordering patterns and highlights the importance of the EHR in providing decision support, guiding ‘default’ ordering behavior, and conserving resources.
Many groups have employed pleocytosis criteria to prevent providers from ordering virus-specific PCRs in clinical contexts with a low pre-test probability.1-3 Our results indicate these PCRs are rarely positive, consistent with work from other groups.1-3 The approach we tested capitalizes on the fast turnaround time of the M/E panel and the high diagnostic value of a positive test for HSV and VZV (treatable and common causes of CNS infections). 9 Moreover, our intervention does not delay care because the recommended ‘first-pass’ studies all result within hours whereas the virus-specific PCRs are run 2-3 times per week. Clinicians remain able to order virus-specific PCR tests if their suspicion for an infection is high despite negative results on the first round of test results (ie an immunocompromised patient, though we suggest ‘adding on’ these tests).
These results may not inform de-escalation of empiric antimicrobial coverage. Although deescalating acyclovir after a negative M/E panel may be reasonable if clinical suspicion is low (or another cause is identified), 9 the M/E panel is likely less sensitive for HSV compared with virus-specific PCRs.10,11 One disadvantage of a limited workup is that patients with 2 infectious etiologies may have one missed, although epidemiologic data indicate that patients with 2 simultaneous causes of meningitis or encephalitis are rare.13,14
We found that most LPs performed using the inpatient LP order set were performed by neurology providers although many cases of HSV were diagnosed by non-neurological services. This observation underscores the relevance of pre-test probability in guiding test selection, as Emergency Department personnel are often performing the LP in acute cases of meningitis and encephalitis.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Kyle A. Lyman https://orcid.org/0000-0002-3309-7211
Zachary D. Threlkeld https://orcid.org/0000-0002-8931-5468
Carl A. Gold https://orcid.org/0000-0002-4868-4152
References
- 1.Majed B, Zephir H, Pichonnier-Cassagne V, et al. Lumbar punctures: use and diagnostic efficiency in emergency medical departments. Int J Emerg Medicine. 2009;2:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roa PL, Alonso R, Egea Vde, Usubillaga R, Muñoz P, Bouza E. PCR for Detection of Herpes Simplex Virus in Cerebrospinal Fluid: Alternative Acceptance Criteria for Diagnostic Workup. J Clin Microbiol. 2013;51:2880-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilen CB, Monaco CL, Hoppe-Bauer J, Jackups R, Bucelli RC, Burnham C-AD. Criteria for Reducing Unnecessary Testing for Herpes Simplex Virus, Varicella-Zoster Virus, Cytomegalovirus, and Enterovirus in Cerebrospinal Fluid Samples from Adults. J Clin Microbiol. 2015;53:887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison AR, Jones MC, Makowski CT, et al. Evaluation of the selection of cerebrospinal fluid testing in suspected meningitis and encephalitis. Diagn Micr Infec Dis. 2022;102:115571. [DOI] [PubMed] [Google Scholar]
- 5.Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27:583-586. [DOI] [PubMed] [Google Scholar]
- 6.Eaton KP, Levy K, Soong C, et al. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern Med. 2017;177:1833. [DOI] [PubMed] [Google Scholar]
- 7.Munigala S, Jackups RR, Poirier RF, et al. Impact of order set design on urine culturing practices at an academic medical centre emergency department. BMJ Qual Saf. 2018;27:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan DJ, Malani P, Diekema DJ. Diagnostic Stewardship—Leveraging the Laboratory to Improve Antimicrobial Use. JAMA. 2017;318:607. [DOI] [PubMed] [Google Scholar]
- 9.Broadhurst MJ, Dujari S, Budvytiene I, Pinsky BA, Gold CA, Banaei N. Utilization, Yield, and Accuracy of the FilmArray Meningitis/Encephalitis Panel with Diagnostic Stewardship and Testing Algorithm. J Clin Microbiol. 2020;58:e00311-e00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trujillo-Gómez J, Tsokani S, Arango-Ferreira C, et al. Biofire FilmArray Meningitis/Encephalitis panel for the aetiological diagnosis of central nervous system infections: A systematic review and diagnostic test accuracy meta-analysis. EClinicalMedicine. 2022;44:101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindström J, Elfving K, Lindh M, Westin J, Studahl M. Assessment of the FilmArray ME panel in 4199 consecutively tested cerebrospinal fluid samples. Clin Microbiol Infec. 2022;28:79-84. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan KV. Diagnostic Stewardship in Clinical Microbiology, Essential Partner to Antimicrobial Stewardship. Clin Chem. 2021;68:75-82. [DOI] [PubMed] [Google Scholar]
- 13.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835-844. [DOI] [PubMed] [Google Scholar]
- 14.Wilson MR, Sample HA, Zorn KC, et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. New Engl J Med. 2019;380:2327-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]