Abstract
Abstract
A well‐coordinated facilitation–inhibition control of motor neuronal persistent inward currents (PICs) via diffuse neuromodulation and local inhibition is essential to ensure motor units discharge at required times and frequencies. Present best estimates indicate that PICs are reduced in older adults; however, it is not yet known whether PIC facilitation–inhibition control is also altered with ageing. We investigated the responses of PICs to (i) a remote handgrip contraction, which is believed to diffusely increase serotonergic input onto motor neurones, and (ii) tendon vibration of the antagonist muscle, which elicits reciprocal inhibition, in young and older adults. High‐density surface electromyograms were collected from soleus and tibialis anterior of 18 young and 26 older adults during triangular‐shaped plantar and dorsiflexion contractions to 20% (handgrip experiments) and 30% (vibration experiments) of maximum torque (rise‐decline rate of 2%/s). A paired‐motor‐unit analysis was used to calculate ∆F, which is assumed to be proportional to PIC strength. ΔF increased in both soleus (0.55 peaks per second (pps), 16.0%) and tibialis anterior (0.42 pps, 11.4%) after the handgrip contraction independent of age. Although antagonist tendon vibration reduced ΔF in soleus (0.28 pps, 12.6%) independent of age, less reduction was observed in older (0.42 pps, 10.7%) than young adults (0.72 pps, 17.8%) in tibialis anterior. Our data indicate a preserved ability of older adults to amplify PICs following a remote handgrip contraction, during which increased serotonergic input onto the motor neurones is expected, in both lower leg muscles. However, PIC deactivation in response to reciprocal inhibition was impaired with ageing in tibialis anterior despite being preserved in soleus.
Key points
Motor neuronal persistent inward currents (PICs) are facilitated via diffuse neuromodulation and deactivated by local inhibition to ensure motor units discharge at required times and frequencies, allowing normal motor behaviour.
PIC amplitudes appear to be reduced with ageing; however, it is not known whether PIC facilitation–inhibition control is also altered.
Remote handgrip contraction, which should diffusely increase serotonergic input onto motor neurones, facilitated PICs similarly in both soleus and tibialis anterior of young and older adults.
Antagonist tendon vibration, which induces reciprocal inhibition, reduced PICs in soleus in both young and older adults but had less effect in tibialis anterior in older adults.
Data from lower‐threshold motor units during low‐force contractions suggest that PIC facilitation is preserved with ageing in soleus and tibialis anterior. However, the effect of reciprocal inhibition on the contribution of PICs to motor neurone discharge seems reduced in tibialis anterior but preserved in soleus.
Keywords: ageing, ΔF , motor unit, motoneuron, reciprocal inhibition
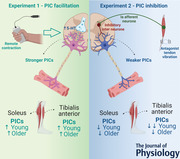
Abstract figure legend Motor neuronal persistent inward currents (PICs) are facilitated via diffuse neuromodulation and deactivated by local inhibition. In Experiment 1 (left panel), remote handgrip contraction, which should diffusely increase serotonergic input onto motor neurones, was used to amplify soleus and tibialis anterior PICs. PICs were facilitated similarly in both soleus and tibialis anterior of young and older adults. In Experiment 2, antagonist (soleus and tibialis anterior) tendon vibration, which induces reciprocal inhibition, was used to inhibit PICs in tibialis anterior and soleus, respectively. PICs in soleus reduced similarly in both young and older adults but had less effect in tibialis anterior in older adults. Figure was created with BioRender.com.

Introduction
Persistent inward currents (PICs), generated by voltage‐gated sodium and calcium channels within the motor neurones, enable amplification and prolongation of excitatory synaptic input (Heckman et al., 2005). Motor neurones can adjust PIC strength via a fine control between neuromodulation and inhibition according to the desired task demands (Heckman et al., 2005). This neuromodulatory control of PICs is dictated by the amount of serotonin and noradrenaline released from the brainstem nuclei and delivered onto the motor neurone dendrites, activating slow activating L‐type Ca2+ and fast activating persistent Na+ currents (Heckman et al., 2008). It has also been suggested that serotonergic projections to the spinal cord vary their activity in proportion to motor output (Jacobs et al., 2002). Thus, the stronger PIC facilitation observed in higher intensity activities (Orssatto, Mackay et al., 2021) could theoretically result from a greater serotonergic release onto the motor neurones (Heckman et al., 2005; Lee & Heckman, 1999, 2000). However, descending serotonergic projections are highly diffuse throughout the spinal cord (Heckman et al., 2008), so activation of a specific muscle triggers excitation across diverse muscle groups, including those not involved in the desired tasks (Heckman et al., 2008; Wei et al., 2014). For example, it has been shown that a remote contraction with a leg muscle group triggers a serotonin‐mediated increase in motor neuronal gain in hand muscles (Wei et al., 2014). Alternatively, deactivation of undesired PICs in specific motor neurones can be achieved by local inhibitory circuits in the spinal cord. PICs are highly sensitive to inhibition and are thus turned off by inhibitory input (Hounsgaard et al., 1988; Hultborn et al., 2003; Kuo et al., 2003; Mesquita et al., 2022; Pearcey et al., 2022; Revill & Fuglevand, 2017). For example, reciprocal inhibition can deactivate PICs from the antagonist muscles (Hyngstrom et al., 2007; Mesquita et al., 2022; Pearcey et al., 2022; Vandenberk & Kalmar, 2014), avoiding undesirable coactivations. Thus, a well‐coordinated facilitation–inhibition control of PICs via diffuse activation (i.e. facilitation) and local deactivation (i.e. inhibition) is essential to ensure motor units discharge at desired times and frequencies, allowing normal motor behaviour (Heckman et al., 2008).
Age‐related alterations within the nervous system (Manini et al., 2013; Orssatto et al., 2018) contribute to the impairments in movement control and force production observed in older individuals (Suetta et al., 2019). Recent estimates suggest that reduced motor neuronal PIC strength plays a role in these impairments (Hassan et al., 2021; Orssatto, Borg et al., 2021). However, it is not known whether PIC neuromodulation–inhibition control is also altered with ageing. Older adults present dysfunctions within the monoaminergic system and inhibitory circuits that could contribute to both inefficient monoaminergic neuromodulation and localised effects of inhibition on PICs (Hortobágyi et al., 2006; Johnson et al., 1993; Kido et al., 2004; Ko et al., 1997; Liu et al., 2020; Míguez et al., 1999; Shibata et al., 2006; Steinbusch et al., 2021), hence potentially contributing to impairments in motor control in this population. Detrimental effects of ageing on the monoaminergic system as well as chronic inflammation reduce serotonin and noradrenaline secretions and thus input onto the motoneurones (Johnson et al., 1993; Ko et al., 1997; Liu et al., 2020; Míguez et al., 1999; Shibata et al., 2006; Steinbusch et al., 2021), which would impair neuromodulation and attenuate PIC facilitation. With respect to inhibitory circuits, older adults have reduced cortical and spinal reciprocal inhibition (Hortobágyi et al., 2006; Kido et al., 2004), which could impair PIC inhibition, generating undesired muscle contractions such as the observed age‐related increases in antagonist coactivation (Hortobágyi & Devita, 2006; Hortobágyi et al., 2009; Macaluso et al., 2002). Exploring the dynamics of PIC neuromodulation and inhibition in older adults could therefore provide important insight into factors underpinning impaired movement control (e.g. locomotion) in this population.
In the present study, the responses of PICs to (i) a remote handgrip contraction, which is believed to diffusely increase serotonergic release onto motor neurones (Heckman et al., 2008; Wei et al., 2014), and (ii) vibration of the antagonist muscle's tendon, which induces reciprocal inhibition (Pearcey et al., 2022), were estimated in soleus and tibialis anterior of young and older adults using the gold‐standard paired motor unit technique (Gorassini et al., 2002; Vandenberk & Kalmar, 2014). To answer these questions, the study involved two experiments. In Experiment 1 we estimated PICs after a remote contraction with upper limb muscles not involved in the soleus‐ and tibialis anterior‐targeting tasks, theoretically inducing increases in serotonergic input onto the motor neurones by taking advantage of their diffuse descending monoaminergic projections into the spinal cord (Heckman et al., 2008; Wei et al., 2014). In Experiment 2, we estimated PIC strength while activating a well‐known disynaptic reciprocal inhibition circuit using tendon vibration of the antagonist muscle (Pearcey et al., 2022), which activates Ia inhibitory interneurones via antagonist Ia afferent stimulation by stimulating its muscle spindles (Burke et al., 1976; Grande & Cafarelli, 2003; Pearcey et al., 2022). We hypothesised that (i) older adults would present smaller PIC increases than young adults in response to a remote handgrip contraction, and (ii) older adults would present reduced attenuation of PICs in response to reciprocal inhibition, irrespective of the tested muscles. Soleus and tibialis anterior were chosen in this study due to their important antagonist co‐involvement in postural stability and locomotion (Laughton et al., 2003; Hortobágyi & Devita, 2006; Polcyn et al., 1998).
Methods
Participants and ethical procedures
Eighteen young adults aged 18−35 years and 26 non‐sarcopenic older adults aged ≥65 years volunteered to the present study. One young and one older adult were excluded from the study because no motor units were identified in soleus and tibialis anterior. Data from 17 young adults (eight women, 29 ± 5 years, dorsiflexion peak torque 41 ± 14 N m, and plantar flexion peak torque 156 ± 47 N m) and 25 older adults (14 women, 70 ± 4 years, dorsiflexion peak torque 29 ± 7 N m, and plantar flexion peak torque 85 ± 32 N m) were analysed. The same participants were tested in a previous study where these characteristics and additional participant data were reported (Orssatto, Borg et al., 2021). More older adults were recruited due to the potential for a smaller motor unit yield due to age‐related motor neurone loss (denervation and reinnervation cycle) (Hepple & Rice, 2016). Thicker fat tissue between the muscle and electrodes was also expected, which could potentially reduce the motor unit yield (Oliveira et al., 2022). The participants had no history of neurological disorders, were free of lower limb musculoskeletal injuries, and were not taking medications that could influence the monoaminergic system, including serotonin or noradrenaline modulators (e.g. beta‐blockers and serotonin reuptake inhibitors). They were instructed to not consume caffeinated foods or beverages 24 h before the testing session. The participants had already completed a previous study in our laboratory from other tests conducted within the same visit, before the procedures of the present study were imposed. Sarcopenia status was screened according to the European Working Group on Sarcopenia in Older People (EWGSOP2) recommendations (Cruz‐Jentoft et al., 2019). No participants had low handgrip strength (<27 kg for men or <16 kg for women) and low appendicular skeletal muscle mass (<20 kg for men and <15 kg for women), indicating they were not sarcopenic. The data related to sarcopenia assessment can be found in our previous study (Orssatto, Borg et al., 2021). This study was approved by the University Human Research Ethics Committee (reference number 1900000634), and all participants gave written informed consent before participating. Data collection was conducted during the COVID‐19 pandemic and all safety procedures followed the local state government policies.
Study design and testing procedures
Participants visited the laboratory on a single occasion. Initially, participants performed a warm‐up and three plantar flexion, dorsiflexion and handgrip maximal voluntary isometric contractions (MVC, ∼3 s with 30 s rest intervals) and were familiarised to the submaximal ramped contractions. Subsequently, they performed four submaximal ramp‐shaped contractions, which have been already analysed and the data published (Orssatto, Borg et al., 2021). After 10 min of rest, the procedures from the present study were conducted. The present cross‐sectional study was divided into two experiments testing plantar and dorsiflexion tasks, which are described below. In Experiments 1 and 2, participants were seated upright in the chair of an isokinetic dynamometer (Biodex System 4, Biodex Medical system, Shirley, NY, USA) with the knee fully extended (0°) and ankle in anatomical position (0°).
Experiment 1 – PIC facilitation
Participants performed two sets of two ramp‐shaped contractions to 20% of their peak torque at a rate of torque rise and decline of 2%/s (10 s up and 10 s down), interspersed by an ipsilateral remote handgrip contraction or resting control condition (Fig. 1A ). Twenty percent of peak torque was used because it is known that ∆F obtained at this intensity has not reached a ceiling, so it would be possible to observe intervention‐dependent increases. Previous studies reported increases in ∆F, from 20% of peak torque contractions, both in tibialis anterior of young adults (Orssatto, Mackay et al., 2021; Udina et al., 2010) and soleus of older adults (Orssatto, Rodrigues et al., 2022). Contracting a muscle unrelated to the tested task is a non‐invasive method to induce serotonergic‐mediated gain on the tested muscle (Wei et al., 2014). The remote handgrip contraction was preceded by a 10 s preparation period, followed by a 30 s contraction to 40% of their maximal handgrip force. These parameters were based on pilot testing, which showed that 40% was the highest contraction intensity that could be universally sustained during 30 s. Participants were asked to avoid any movement (i.e. muscle contraction) during the resting control condition, which lasted 40 s. The subsequent ramp‐shaped contractions were performed immediately after the remote handgrip or resting control conditions (i.e. remote contraction was ceased before plantar‐ or dorsiflexion contractions commenced so dual tasking was avoided). The order in which each condition was performed and in which each muscle was tested was randomised. Participants received real‐time visual torque–trace feedback during each contraction and were instructed to follow the torque path displayed on a 58‐cm computer monitor. When an abrupt increase or decrease in torque was observed (i.e. the torque trajectory was not closely followed), the whole trial was excluded and repeated 2 min later.
Figure 1. Design of Experiments 1 and 2.
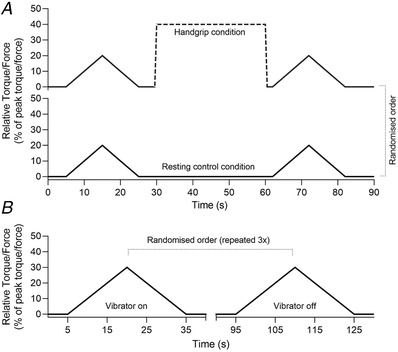
A, Experiment 1 design with the handgrip condition shown at top and control condition at bottom. B, Experiment 2 design. In both experiments, PICs were estimated during submaximal ramp‐shaped contractions with rate of torque rise and decline of 2%/s. The contraction intensities were 20% of peak torque (20 s total duration) and 30% of peak torque (30 s total duration) for Experiments 1 and 2, respectively.
Experiment 2 – PIC inhibition
Participants performed six ramp‐shaped contractions to 30% of their peak torque at a rate of torque rise and decline of 2%/s (15 s up and 15 s down). Thirty percent of peak torque was used because (i) soleus ∆F obtained at this intensity has the potential to be reduced (Orssatto, Mackay et al., 2021), and (ii) reductions in soleus and tibialis anterior ∆F in response to reciprocal inhibition have been observed in young adults at this intensity (Pearcey et al., 2022). Three contractions were performed with high‐frequency vibration applied to the tendon of the antagonist muscles and three with the vibrator device turned off (i.e. control condition). Conditions were alternated in a randomised order, interspersed by 1‐min rest intervals (Fig. 1B ). Tendon vibration (115 Hz, Vibrasens, Technoconcept, Saint_Maurice, France) was applied to the Achilles tendon during the dorsiflexion contractions and to the tibialis anterior tendon during the plantar flexion contractions. The vibrator device was firmly strapped onto the leg and no discomfort was reported by the participants. However, the amount of pressure applied on the skin was not quantified. Vibration commenced 10 s before the start of the ramp‐shaped contraction and continued until 2 s after the torque trace returned to baseline, and was thus imposed through the duration of the ramp contraction.
Surface electromyography
Skin preparation included shaving, abrasion and cleansing each site with 70% isopropyl alcohol. Two semi‐disposable 32‐channel electrode grids with a 10‐mm interelectrode distance (ELSCH032NM6, OT Bioelettronica, Turin, Italy) were placed over the medial and lateral portions of soleus (either side of the Achilles tendon) and another two electrode grids were placed over the superior and inferior aspect of tibialis anterior using a bi‐adhesive foam layer and conductive paste (Ten20, Weaver and Company, Aurora, CO, USA). A dampened strap electrode (WS2, OT Bioelettronica) was positioned around the ankle joint as a ground electrode. Surface electromyograms (sEMG) were recorded during the submaximal ramp‐shaped contractions. The sEMG signals were acquired in monopolar mode, amplified (256×), band‐pass filtered (10−500 Hz), and converted to a digital signal at 2048 Hz by a 16‐bit wireless amplifier (Sessantaquattro, OT Bioelettronica) using OTBioLab+ software (version 1.3.0., OT Bioelettronica) before being stored for offline analysis.
Motor unit analyses
Motor unit identification
The recorded data were processed offline using DEMUSE software (Holobar & Zazula, 2007). For each muscle from Experiment 1, only the pair of ramp‐shaped contractions yielding the lowest deviation from torque trajectory were analysed for the handgrip or resting control conditions. The same was adopted for Experiment 2, in which only the motor units from one pair of ramp‐shaped contractions (vibration and control conditions) yielding the lowest deviation from torque trajectory were analysed. If pairs of contractions presented a similar torque trajectory, the pair with the highest number of identified motor units was analysed. High‐density sEMG signals were band‐pass filtered (20−500 Hz) with a second‐order, zero‐lag Butterworth filter. Thereafter, a blind source separation method, the convolutive kernel compensation (CKC) method, was used for signal decomposition (Holobar & Zazula, 2007; Holobar et al., 2014) from each triangular contraction. CKC yields the filters of individual motor units that, when applied to high‐density sEMG signals, estimate the motor unit spike trains (Holobar & Zazula, 2007; Holobar et al., 2014). The motor unit filters were used to identify the same motor unit across different time points. In Experiment 1, motor units were tracked before and after the handgrip or resting control conditions, but not between each condition. In Experiment 2, motor units were tracked between the vibrator on and vibrator off conditions. The motor unit filters identified by convolutive kernel compensation at individual contractions on each time point were applied to the concatenated high‐density sEMG signals recorded at other time points. Afterwards, motor unit filters identified from each time point were applied to the concatenated recordings (Frančič & Holobar, 2021, 2022) yielding the motor unit spike trains of all the identified motor units across all the concatenated time points. After removing motor unit duplicates simultaneously identified from two time points, a trained investigator manually inspected motor unit spike trains and edited the discharge patterns of the motor units. Only motor units visually inspected and with a pulse‐to‐noise ratio equal to or greater than 30 dB were kept for further analysis (Holobar et al., 2014).
Estimation of PIC strength (ΔF) and peak discharge rates
The observed discharge events for each motor unit were converted into instantaneous discharge rates and fitted into a fifth‐order polynomial function. PIC strengths were estimated using the paired motor unit analysis (Gorassini et al., 2002). Motor units with a lower recruitment threshold (i.e. control units) were paired with higher recruitment threshold motor units (i.e. test units). ΔF was calculated as the change in discharge rates of the control motor unit from the moment of recruitment to the moment of de‐recruitment of the test unit (Gorassini et al., 2002). Motor unit pairs were composed of motor units with rate‐to‐rate correlations between the smoothed discharge rate polynomials of r ≥ 0.7, and the test units were recruited at least 1.0 s after the control units (Gorassini et al., 2002; Hassan et al., 2020). ΔF values obtained for each control unit were averaged to obtain a single ΔF for each test motor unit. ΔF values for the motor units tracked over time were derived from the same pairs of motor units on each condition (i.e. Experiment 1, between before and after handgrip or resting control conditions, and Experiment 2, between vibrator on and vibrator off conditions). The maximum value obtained from the polynomial curve was considered the peak discharge rate. The relative torque (%) produced at the time in each motor unit was recruited was considered the recruitment threshold. Figure 2 shows an example of paired motor unit analysis used to calculate ΔF from a single test unit using three control units.
Figure 2. Data illustrating the ΔF calculation using paired motor unit analysis.
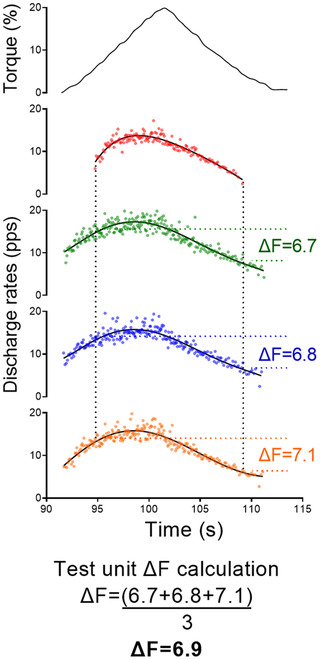
The top panel shows the torque traces for contractions to 20% of the participant's dorsiflexion maximal voluntary torque. The subsequent panels display a tibialis anterior test motor unit (red colour) and three control units (green, blue and orange colours). The black continuous lines are the fifth‐order polynomial fits for the control units. The ΔF values obtained from each control unit were averaged, resulting in a single value for the test unit. [Colour figure can be viewed at wileyonlinelibrary.com]
Data analyses
In Experiment 1, separate linear mixed‐effect models were used for each muscle to compare ΔF, peak discharge rates and recruitment thresholds between conditions (i.e. control and handgrip), age groups (i.e. older and young adults), and over time (i.e. before and after handgrip or control conditions), used as fixed factors (Yu et al., 2021). Additional linear mixed‐effect models were used to compare ΔF mean differences (after – before handgrip or control conditions) between conditions and age groups to remove the time factor from the model, and results are presented in Supplementary Material 1. In Experiment 2, linear mixed‐effect models were used to compare ΔF, peak discharge rates and recruitment thresholds between conditions (i.e. control and vibrator), used as fixed factors (Yu et al., 2021). For both experiments, soleus and tibialis anterior were fitted to separate statistical models. Single motor units were treated as repeated measures, nested according to each participant, and a random intercept was included for each participant in the study to account for the correlation between repeated observations on each individual (i.e. 1| participant/motor unit ID). When a significant effect was observed, Bonferroni post hoc correction was adopted for pairwise comparison. The effect sizes derived from the F ratios were calculated with the omega‐square (ω2) method (0−0.01, very small; 0.01−0.06, small; 0.06−0.14, moderate; and >0.14, large) (Lakens, 2013). Intraclass correlation coefficient (ICC, model 2, k) and standard error of the mean (SEM) were calculated between ΔF before and after the control condition from Experiment 1. The standardised difference (Cohen's d) between time points was also calculated using the population standard deviation from each respective linear mixed‐effects model as the denominator (Lenth et al., 2021). Repeated‐measures correlations were used to determine the association between changes in ΔF and changes in discharge rates and recruitment thresholds in both experiments 1 and 2 (Bakdash & Marusich, 2017). Correlation magnitude was interpreted as: r < 0.1 trivial; 0.1–0.3 small; 0.3–0.5 moderate; 0.5–0.7 large; 0.7–0.9 very large; and >0.9 nearly perfect. All analyses were completed in R (version 4.0.5) using the RStudio environment (version 1.4.1717). Linear mixed‐effects models were fitted using the lmerTest package (Kuznetsova et al., 2017). Estimated marginal mean differences and 95% confidence intervals between time points were determined using the emmeans package (Lenth et al., 2021). The repeated‐measures correlation coefficients were determined with the rmcorr package (Bakdash & Marusich, 2017). Significant difference was accepted at P ≤ 0.05. All descriptive data are presented as mean (95% confidence interval lower and upper limits), unless indicated differently. The dataset and R code can be found at https://github.com/orssatto/PICs‐ageing_2.0.
Results
Motor unit identification
In Experiment 1, 201 soleus motor units from 21 older adults and 110 soleus motor units from 14 young adults were identified by the decomposition algorithm that could be matched before and after the control condition, resulting in 78 and 64 test units, respectively. One hundred and eighty‐seven soleus motor units were matched before and after the handgrip contraction for 19 older adults and 101 for 12 young adults, resulting in 79 and 43 test units, respectively. Three hundred and twenty‐six tibialis anterior motor units from 16 older adults and 262 from 15 young adults were matched before and after the control condition, resulting in 154 and 132 test units, respectively. Two hundred and fifty‐three tibialis anterior motor units from 14 older adults and 222 from 13 young adults were matched before and after the control condition, resulting in 110 and 116 test units, respectively. In Experiment 2, 97 soleus motor units from 15 older adults and 60 motor units from eight young adults were matched between control and vibration conditions, resulting in 42 and 35 test units respectively. Two hundred and forty‐six tibialis anterior motor units from 13 older adults and 215 motor units from 13 young adults were matched between control and vibration conditions, resulting in 143 and 147 test units, respectively. Motor units sample median and interquartile range per experiment, group and conditions are presented in Table 1.
Table 1.
Total and test motor unit sample medians (interquartile range) for each group and condition used in Experiments 1 and 2
| Experiment 1 | ||||
|---|---|---|---|---|
| Older adults | Young adults | |||
| Control | Handgrip | Control | Handgrip | |
| Soleus motor units | 8 (7, 10) | 8 (6, 11) | 7 (5, 9) | 8 (7, 9) |
| Soleus test units (ΔF) | 3 (2, 4) | 2 (2, 7) | 5 (3, 5) | 3 (2, 4) |
| Tibialis anterior motor units | 21 (16, 26) | 17 (14, 23) | 17 (13, 23) | 17 (14, 22) |
| Tibialis anterior test units (ΔF) | 10 (7, 14) | 10 (6, 10) | 8 (5, 13) | 8 (5, 13) |
| Experiment 2 | ||||
| Older adults | Young adults | |||
| Soleus motor units | 6 (5, 7) | 5 (4, 8) | ||
| Soleus test units (ΔF) | 3 (1, 4) | 2 (1, 5) | ||
| Tibialis anterior motor units | 19 (15, 26) | 18 (11, 20) | ||
| Tibialis anterior test units (ΔF) | 11 (7, 14) | 14 (5, 17) | ||
The presented numbers represent the quantity of motor units tracked over time (before and after each condition) in Experiment 1 and tracked between conditions (vibration and control) in Experiment 2.
Experiment 1 – PIC facilitation
In summary, ΔF and peak discharge rates were increased in motor units of young and older adults following the handgrip condition but not the control condition. These results were observed in both soleus and tibialis anterior, independent of age (Figs 3 and 4). Recruitment threshold did not change in soleus, independent of age, but increased in tibialis anterior in older adults only. Descriptive statistics for each group, condition, time point and muscle are presented in Table 2.
Figure 3. Soleus ΔF outcomes in control and handgrip conditions for older and young adults.
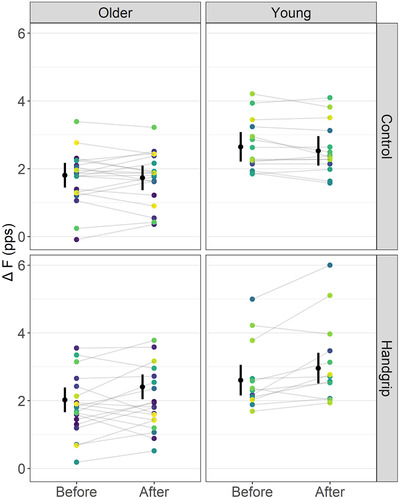
Note that ΔF remained unchanged before and after the control condition but increased in the handgrip condition in both older and young adults. The mean (black circle) and 95% confidence intervals are offset to the left. Individual data points (average ΔF per participant) are coloured by participants. pps, peaks per second. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4. Tibialis anterior ΔF outcomes in control and handgrip conditions for older and young adults.
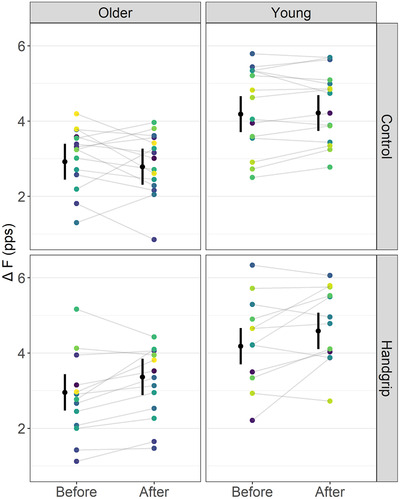
Note that ΔF remained unchanged before and after the control condition but increased in the handgrip condition in both older and young adults. The mean (black circle) and 95% confidence intervals are offset to the left. Individual data points (average ΔF per participant) are coloured by participants. pps, peaks per second. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 2.
Estimated marginal means and mean differences (95% confidence interval lower and upper limits) for ΔF, peak discharge rates, and recruitment thresholds before and after control or handgrip conditions in young and older adults and for soleus and tibialis anterior
| ΔF (pps) | Peak discharge rates (pps) | Recruitment threshold (% of peak torque) | |
|---|---|---|---|
| Older adults – soleus | |||
| Before control | 1.81 (1.44, 2.17) | 8.75 (8.17, 9.32) | 8.11 (6.92, 9.30) |
| After control | 1.73 (1.36, 2.09) | 8.62 (8.05, 9.20) | 8.39 (7.20, 9.57) |
| After – before control | −0.08 (−0.37, 0.21) | −0.13 (−0.39, 0.14) | 0.28 (−0.76, 1.31) |
| Before handgrip | 2.02 (1.66, 2.39) | 8.54 (7.96, 9.12) | 8.66 (7.47, 9.85) |
| After handgrip | 2.41 (2.04, 2.77) | 9.00 (8.42, 9.57) | 9.01 (7.82, 10.20) |
| After – before handgrip | 0.39 (0.10, 0.67) | 0.46 (0.20, 0.72) | 0.35 (−0.68, 1.38) |
| Young adults – soleus | |||
| Before control | 2.64 (2.21, 3.08) | 10.65 (9.95, 11.36) | 9.20 (7.79, 10.61) |
| After control | 2.53 (2.09, 2.96) | 10.75 (10.04, 11.45) | 9.03 (7.62, 10.45) |
| After – before control | −0.12 (−0.44, 0.20) | 0.10 (−0.19. 0.39) | −0.17 (−1.31, 0.98) |
| Before handgrip | 2.60 (2.15, 3.06) | 10.57 (9.85, 11.28) | 9.54 (8.06, 11.02) |
| After handgrip | 2.96 (2.51, 3.41) | 10.91 (10.20, 11.63) | 8.42 (6.94, 9.90) |
| After – before handgrip | 0.35 (−0.04, 0.74) | 0.34 (−0.01, 0.70) | −1.12 (−2.51, 0.28) |
| Older adults – tibialis anterior | |||
| Before control | 2.92 (2.44, 3.40) | 12.14 (11.09, 13.19) | 10.39 (8.94, 11.84) |
| After control | 2.79 (2.31, 3.26) | 11.89 (10.84, 12.95) | 10.88 (9.43, 12.33) |
| After – before control | −0.13 (−0.39, 0.13) | −0.25 (−0.60, 0.10) | 0.49 (−0.17, 1.15) |
| Before handgrip | 2.95 (2.47, 3.44) | 12.04 (10.98, 13.10) | 9.71 (8.24, 11.18) |
| After handgrip | 3.36 (2.88, 3.85) | 12.22 (11.16, 13.28) | 11.36 (9.88, 12.83) |
| After – before handgrip | 0.41 (0.10, 0.72) | 0.18 (−0.23, 0.59) | 1.65 (0.80, 2.49) |
| Young adults – tibialis anterior | |||
| Before control | 4.18 (3.71, 4.66) | 14.75 (13.71, 15.79) | 11.36 (9.91, 12.80) |
| After control | 4.21 (3.74, 4.69) | 14.71 (13.67, 15.75) | 10.79 (9.34, 12.23) |
| After – before control | 0.03 (−0.25, 0.30) | −0.04 (−0.41, 0.33) | −0.57 (−1.29, 0.15) |
| Before handgrip | 4.18 (3.70, 4.66) | 14.12 (13.08, 15.17) | 11.56 (10.10, 13.02) |
| After handgrip | 4.59 (4.10, 5.07) | 14.66 (13.62, 15.75) | 11.05 (9.59, 12.51) |
| After – before handgrip | 0.41 (0.10, 0.71) | 0.53 (0.12, 0.94) | −0.51 (−1.31, 0.29) |
Estimates of PICs (ΔF)
For soleus, a time by condition interaction on ΔF (F = 19.27, ω2 = 0.05, P < 0.001), but not a time by condition by age interaction (F < 0.01, ω2 < 0.001, P = 0.967), was detected. ΔF did not change over time in the control condition (mean difference (MD) = −0.10 (−0.28, 0.09) pps, d = −0.17 (−0.41, 0.08), P = 0.17) but increased in the handgrip condition (MD = 0.37 (0.16, 0.57) pps, d = 0.62 (0.35, 0.90), P < 0.001). Before the interventions, ΔF was not different between the control and handgrip conditions (MD = −0.09 (−0.30, 0.13), d = −0.15 (−0.43, 0.14), P = 0.292). However, ΔF was higher in the handgrip condition than the control condition after the intervention (MD = 0.55 (0.34, 0.77), d = 0.932 (0.64, 1.22), P < 0.001) (Fig. 3). ICC for ΔF measured before and after the control condition was 0.968 (0.944, 0.982) and SEM was 0.219 pps.
For tibialis anterior, there was a time by condition interaction on ΔF for tibialis anterior (F = 23.81, ω2 = 0.03, P < 0.001) but not a time by condition by age interaction (F = 0.76, ω2 < 0.001, P = 0.385). ΔF did not change over time in the control condition (MD = −0.05 (−0.21, 0.11) pps, d = −0.07 (−0.24, 0.10), P = 0.399), but increased in the handgrip condition (MD = 0.42 (0.23, 0.59) pps, d = 0.55 (0.35, 0.74), P < 0.001). Before the interventions, ΔF was not different between control and handgrip conditions (MD = −0.01 (−0.20, 0.17) pps, d = −0.02 (−0.22, 0.18), P = 0.838). However, ΔF was higher in the handgrip than the control condition after the interventions (MD = 0.64 (0.44, 0.84) pps, d = 0.61 (0.40, 0.81), P < 0.001) (Fig. 4). ICC for ΔF measured before and ΔF after the control condition was 0.946 (0.901, 0.971) and SEM was 0.372 pps.
Peak discharge rates
For soleus, a time by condition interaction (F = 18.52, ω2 = 0.05, P < 0.001), but not a time by condition by age interaction (F = 3.02, ω2 < 0.001, P = 0.083), was detected. Peak discharge rates did not change over time in the control condition (MD = −0.02 (−0.18, 0.15) pps, d = −0.03 (−0.27, 0.21), P = 0.812) but increased in the handgrip condition (MD = 0.40 (0.21, 0.59) pps, d = 0.74 (0.47, 1.02), P < 0.001). For tibialis anterior, there was a time by condition interaction (F = 15.57, ω2 = 0.02, P < 0.001) but no time by condition by age interaction (F = 0.345, ω2 < 0.001, P = 0.557). Peak discharge rates did not change over time in the control condition (MD = −0.14 (−0.36, 0.07) pps, d = −0.14 (−0.31, 0.03), P = 0.084) but increased after the handgrip condition (MD = 0.36 (0.11, 0.60) pps, d = 0.36 (0.16, 0.56), P = 0.002), irrespective of age. A small correlation was observed between changes in ∆F and peak discharge rate for soleus (r = 0.22 (0.11, 0.31)) and tibialis anterior (r = 0.21 (0.13, 0.28)).
Recruitment thresholds
For soleus, there was no time by condition by age interaction (F = 1.81, ω2 < 0.001, P = 0.180) nor time by condition interaction (F = 1.33, ω2 < 0.001, P = 0.250). Recruitment threshold remained unchanged before and in both the control (MD = 0.06 (−0.60, 0.71)% of peak torque, d = 0.03 (−0.28, 0.28), P = 0.826) and handgrip (MD = −0.44 (−1.2, 0.32)% of peak torque, d = −0.18 (−0.45, 0.09), P = 0.179) conditions, irrespective of age. For tibialis anterior, there was a time by condition by age interaction (F = 4.79, ω2 < 0.001, P = 0.029). Recruitment threshold increased after handgrip in older (d = 0.84 (0.55, 1.13), P < 0.001) but not in young adults (d = −0.26 (−0.53, 0.01), P = 0.527), and remained unchanged before and after control for both older (P = 0.319) and young adults (P = 0.235). A trivial correlation was observed between changes in ∆F and recruitment threshold for soleus (r = 0.12 (0.02, 0.23)), while no correlation was observed for tibialis anterior (r = 0.03 (−0.05, 0.10)).
Experiment 2 – PIC inhibition
In summary, ΔF decreased in motor units of both young and older adults when vibration was applied to the antagonist tendon. In soleus, these reductions were similar for young and older adults (Fig. 5A ) but in tibialis anterior the magnitude of reduction was smaller for older than young adults (Fig. 5B ). Peak discharge rates remained unchanged in soleus but reduced for tibialis anterior, irrespective of age. Recruitment threshold remained unchanged in soleus, irrespective of age, but increased for young adults only in tibialis anterior. Descriptive statistics for each group, condition and muscle are presented in Table 3.
Figure 5. Soleus (A) and tibialis anterior (B) ΔF outcomes in control and vibration conditions for older and young adults.
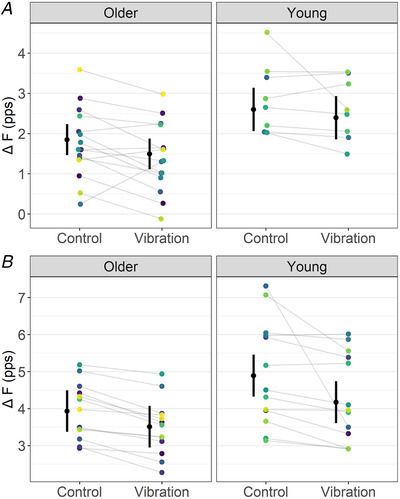
Note that, for soleus, ΔF was reduced during the vibration compared to the control condition, similarly between older and young adults. For tibialis anterior, ΔF was reduced in older adults during the vibration condition, but to a smaller magnitude than young adults (i.e. condition by age interaction). The mean (black circle) and 95% confidence interval are offset to the left. Individual data points (average ΔF per participant) are coloured by participants. pps, peaks per second. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 3.
Estimated marginal means and mean differences (95% confidence interval lower and upper limits) for ΔF, peak discharge rates, and recruitment thresholds for vibration and control conditions
| ΔF (pps) | Peak discharge rates (pps) | Recruitment threshold (% of peak torque) | |
|---|---|---|---|
| Older adults – soleus | |||
| Control | 1.85 (1.46, 2.23) | 9.09 (8.20, 9.98) | 12.15 (9.59, 14.33) |
| Vibration | 1.49 (1.11, 1.88) | 8.93 (8.04, 9.82) | 12.37 (10.18, 14.55) |
| Vibration – Control | −0.36 (−0.65. −0.06) | −0.16 (−0.38, 0.06) | 0.22 (−1.41, 0.35) |
| Young adults – soleus | |||
| Control | 2.60 (2.06, 2.23) | 10.63 (9.40, 11.85) | 15.01 (12.01, 18.00) |
| Vibration | 2.39 (1.86, 2.93) | 10.39 (9.17, 11.61) | 14.58 (11.58, 17.57) |
| Vibration – Control | −0.21 (−0.53, 0.12) | −0.24 (−0.48, 0.01) | −0.43 (−1.73, 0.87) |
| Older adults – tibialis anterior | |||
| Control | 3.93 (3.37, 4.49) | 13.15 (12.26, 14.05) | 11.62 (9.84, 13.40) |
| Vibration | 3.51 (2.95, 4.07) | 12.47 (11.58, 13.37) | 11.47 (9.69, 13.25) |
| Vibration – Control | −0.42 (−0.67, −0.17) | −0.72 (−0.97, −0.47) | −0.15 (−0.88, 0.57) |
| Young adults – tibialis anterior | |||
| Control | 4.89 (4.32, 5.46) | 14.50 (13.60, 15.40) | 14.14 (12.34, 15.95) |
| Vibration | 4.17 (3.61, 4.74) | 13.90 (13.00, 14.80) | 15.33 (13.53, 17.14) |
| Vibration – Control | −0.87 (−1.11, −0.62) | −0.60 (−0.85, −0.35) | −1.19 (−1.90, −0.47) |
Estimates of PICs (ΔF)
For soleus, a condition effect on ΔF (F = 11.16, ω2 = 0.12, P = 0.001), but not a condition by age interaction (F = 0.80, ω2 < 0.001, P = 0.374), was detected. ΔF was lower in the vibration than the control condition, irrespective of age group (MD = 0.28 (0.11, 0.45) pps, d = 0.54 (0.19, 0.89)) (Fig. 5A ). For tibialis anterior, there was a condition by age interaction on ΔF (F = 4.66, ω2 = 0.01, P = 0.032). ΔF reductions in older adults (MD = 0.42 (0.17, 0.67) pps, d = 0.51 (0.26, 0.76)) were smaller than in young adults (MD = 0.72 (0.47, 0.97) pps, d = 0.87 (0.62, 1.11)) (Fig. 5B ). A pair of outlier motor units was identified in the tibialis anterior analysis but excluding them from the model did not remove the evidence of condition by age interaction (F = 3.68, ω2 = 0.01, P = 0.056).
Peak discharge rates
For soleus, a condition effect on peak discharge rates (F = 9.98, ω2 = 0.10, P = 0.002), but not a condition by age interaction (F = 0.41, ω2 < 0.001, P = 0.526), was detected. Peak discharge rates were 0.20 (0.07, 0.32) pps lower in the vibration than the control condition (d = 0.51 (0.17, 0.86)), irrespective of age. For tibialis anterior, a condition effect on peak discharge rates (F = 86.22, ω2 = 0.23, P < 0.001), but not a condition by age interaction (F = 0.34, ω2 < 0.001, P = 0.559), was detected. Peak discharge rates were 0.64 (0.50, 0.78) pps lower in the vibration than control condition (d = 0.77 (0.59, 0.95)) irrespective of age. A small correlation was observed between changes in ∆F and peak discharge rate for soleus (r = 0.22 (0.00, 0.43)) and a large correlation for tibialis anterior (r = 0.57 (0.49, 0.64)).
Recruitment threshold
For soleus, there was no condition effect (F = 0.10, ω2 = 0.01, P = 0.752) nor a condition by age interaction on recruitment threshold (F = 0.95, ω2 < 0.001, P = 0.333). For tibialis anterior, there was a condition by age interaction on recruitment threshold (F = 11.61, ω2 = 0.04, P < 0.001). Recruitment threshold remained unchanged in older adults (d = −0.06 (−0.31, 0.18), P = 0.585) but increased in young adults (d = 0.50 (0.26, 0.74), P < 0.001). No correlation was observed between changes in ∆F and recruitment thresholds for soleus (r = 0.05 (−0.18, 0.27)) or tibialis anterior (r = −0.07 (−0.19, 0.04)).
Discussion
This study tested the ability of older and young adults to both amplify and inhibit motoneuronal PICs, estimated using paired motor unit analyses (i.e. ΔF), in response to a remote handgrip contraction and to reciprocal inhibition, respectively. We previously showed that ΔF was lower in older than young adults, measured in the same cohort of subjects (Orssatto, Borg et al., 2021). However, here we present the first evidence that the abilities of older adults to modulate PICs in response to a remote handgrip contraction, which theoretically increases monoaminergic projections onto the motor neurones, is preserved in both soleus and tibialis anterior. These results are not consistent with our hypothesis that older adults would have a reduced ability to increase ΔF after a remote handgrip contraction. However, we also observed that older adults’ abilities to inhibit ΔF was preserved in soleus but not tibialis anterior, in which they presented attenuated inhibition. These data partially agree with our initial hypothesis that inhibition would be impaired in older adults but clarify that the effect may not be consistent between muscles or muscle groups, which we discuss further below. The novel findings from this study are fundamental to our understanding of the facilitation–inhibition control of PICs in older and young adults.
Experiment 1 – PIC facilitation
Increases in ΔF following the 30 s remote handgrip contraction (at 40% of maximal force) for both soleus (16%) and tibialis anterior (11.4%) were consistent with our initial hypothesis. This PIC facilitation likely results from the increased available serotonin delivered by descending tracts within the spinal cord, which has diffuse input onto motor neurones across diverse muscle groups (Heckman et al., 2008; Wei et al., 2014). This should be predominately attributed to the activity of serotonergic projections to the spinal cord, which is increased along with motor output (Jacobs et al., 2002), rather than noradrenaline, which is more influenced by arousal state (Aston‐Jones et al., 2001). Therefore, the increased availability of serotonin along with its highly diffuse descending projections into the spinal cord is expected to be the main mechanism underpinning the observed PIC facilitation.
Contrary to expectation, young and older adults showed similar PIC facilitation after the sustained handgrip contraction in both soleus and tibialis anterior. One consideration is that this similarity might be limited to relatively light submaximal contractions, such as the ramp contractions in our study which peaked at 20% of maximum torque capacity. The choice of this torque level was largely based on matching the forces that may be exerted during walking or postural sway, and partly based on ensuring optimal motor unit identification (fewer motor unit pairs are detected at higher contraction levels and fatigue is generated by prolonged and high intensity contractions, making the technique less feasible as contraction level increases). However, the magnitude of PIC facilitation depends critically on the level of monoaminergic drive onto the motor neurones (Hounsgaard et al., 1988; Lee & Heckman, 1999, 2000), which in turn is affected by the exerted contraction intensity (Jacobs et al., 2002). Thus, stronger contractions demand greater release of monoamines onto the motor neurones and should enable greater PIC facilitation (Hounsgaard et al., 1988; Lee & Heckman, 1999, 2000; Orssatto, Mackay et al., 2021). Using contractions to 20% of maximum will have prompted only a moderate increase in serotonin concentration, and potentially below some existing ceiling, allowing the observed PIC facilitation in the older group to occur subsequent to the handgrip contraction. We cannot rule out the possibility that higher intensity contractions, providing stronger monoaminergic input to amplify PICs, would have reduced the capacity for the handgrip contraction itself to provide monoaminergic drive above that already provided by the contraction itself, in either the older or the young adults. Nonetheless, a recent study by our group (including a subset of older adults from the present study) showed that non‐strength‐trained older adults were unable to further amplify soleus PICs as plantar flexion contraction intensity increased from 20% to 40% of peak torque (Orssatto, Rodrigues et al., 2022). This is not consistent with the behaviour of increased PIC facilitation observed in young adults when contraction intensity increased from 10% to 30% of peak torque (Orssatto, Mackay et al., 2021), indicating an age‐dependent difference in the capacity to increase PIC strength with contraction intensity. These results are suggestive of a potentially impaired serotonergic input onto the motor neurones that may manifest at higher contraction intensities in older individuals only. Although ΔF values are more difficult to attain in higher‐force contractions, it would be of interest to test PIC facilitation at higher force levels in future experiments.
Experiment 2 – PIC inhibition
We tested the hypothesis that ΔF should decrease during reciprocal inhibition, induced by antagonist tendon vibration, in soleus and tibialis anterior. Our data demonstrate that high‐frequency vibration of tibialis anterior and the Achilles tendon successfully induced reciprocal inhibition, reducing ΔF in soleus by 0.28 pps (12.6%) in both older and young adults, and in tibialis anterior by 0.42 pps (10.7%) in older adults and 0.87 pps (17.8%) in young adults. High‐frequency tendon vibration triggers excitatory drive from Ia afferents via stimulation of muscle spindles, which consequently generates reciprocal inhibition by activating the Ia inhibitory interneurones (Burke et al., 1976). The reductions in ΔF during the reciprocal inhibition condition shown in our data are consistent with findings from animal experiments showing reciprocal inhibition‐evoked PIC reductions induced by in vivo voltage‐clamp techniques in cats (Kuo et al., 2003; Hyngstrom et al., 2007). However, using such techniques, dendritic PICs were reduced by ∼50% in the ankle extensors by imposition of small joint rotations (Hyngstrom et al., 2007) and by ∼69% during tonic nerve electrical stimulation to antagonist muscles (in a standard monoaminergic state) (Kuo et al., 2003). Both of these animal experiments therefore showed a much greater reduction than we observed in the current experiment. Nonetheless, our results are consistent with recent human experiments showing: (i) comparable ΔF reductions of 0.54 ± 0.09 pps in both tibialis anterior (effect size g = 0.49) and soleus (g = 0.26) using a similar protocol of antagonist tendon vibration (Pearcey et al., 2022); (ii) similar ΔF reductions of 0.33 pps (9.8%) in gastrocnemius medialis of young adults after reciprocal inhibition was induced by electrical stimulation of the common peroneal nerve (Mesquita et al., 2022); (iii) inverse correlation between the level of reciprocal inhibition (induced with stimulations to the common peroneal nerve, below motor unit threshold) and ΔF in soleus (Vandenberk & Kalmar, 2014); and (iv) artificial activation of inhibitory reflex by sural nerve stimulation reducing the initial steep increases in discharge rates in tibialis anterior motor neurones during ramped contractions, which is likely modulated by PICs (Revill & Fuglevand, 2017). These data suggest that the magnitude of reciprocal inhibition on PICs may be smaller when assessed by the ΔF method in humans than in direct measurements in animal experiments. This could result from the stronger reciprocal inhibition evoked in animal experiments (Hyngstrom et al., 2007; Kuo et al., 2003) and/or because ΔF only estimates the portion of PICs above the discharging threshold (Gorassini et al., 2002).
We hypothesised that older adults would present smaller ΔF reductions than young adults because reciprocal inhibition is generally considered to be reduced with ageing in both soleus and tibialis anterior (Kido et al., 2004). However, our data show a difference only in tibialis anterior. The weaker inhibition of tibialis anterior in the older adults might be explained by (i) an age‐related alteration in reciprocal inhibition due to a decrease in muscle spindle quantity or changes in their structure, innervation and sensitivity (Henry & Baudry, 2019), (ii) reductions in the total number of nerve fibres, including spindle afferents (Swallow, 1966), and deterioration of Ia afferent pathways (observed in aged rats) (Vaughan et al., 2017), (iii) changes in the transmission efficacy at the Ia inhibitory inter neurone, or (iv) efferent fibre impairment (Geertsen et al., 2017). Although there is a lack of consensus in the literature, reduced transmission efficacy of Ia afferents with ageing (measured indirectly with H‐reflexes) (Klass et al., 2011; Scaglioni et al., 2012, 2003; Škarabot et al., 2019, 2020) could contribute to an impaired reciprocal inhibition of motor neuronal PICs. As a consequence, this could partly underpin the increased tibialis anterior coactivation during important daily tasks such as single leg stance (Kurz et al., 2018) and during gait (Hortobágyi et al., 2009) in older adults, which should be explored in future studies. Another mechanism that should be considered is a possible ceiling effect related to inhibition control. The pattern of the inhibitory commands is altered with ageing (Butchart et al., 1993; Hortobágyi et al., 2006; Kido et al., 2004) and may contribute to the lower ΔF observed in older adults at baseline (Hassan et al., 2021; Orssatto, Borg et al., 2021). As such, additional inhibitory input might have reduced the opportunity for further decrease in ∆F in tibialis anterior of older adults.
The dissimilar response between soleus and tibialis anterior may speculatively be explained by their different functional roles in daily tasks. Both muscles are active during daily living activities, such as upright standing and locomotion (e.g. gait) (Masani et al., 2013; Soames & Atha, 1981). However, soleus is a propulsive muscle and serves an anti‐gravity role, implying that motor units are active for longer and produce greater cumulative force than non‐propulsive flexor muscles (e.g. tibialis anterior) during daily living activities. Additional data supporting this claim include evidence that peak discharge rates are maintained with ageing in well‐used (e.g. hand muscles) and gravity‐loaded muscles (e.g. quadriceps and triceps surae) but decline markedly in lesser used/loaded muscles (e.g. hamstrings and tibialis anterior) (Orssatto, Borg et al., 2022). Also, the estimated number of motor units in soleus seems preserved with ageing, again suggesting selective preservation in this muscle (Dalton et al., 2008). In fact, studies show that disuse can aggravate the deleterious effects of ageing on the nervous system, while trained older adults show a substantial preservation of neural function (Aagaard et al., 2010; Hvid et al., 2018; McGregor et al., 2011; Orssatto et al., 2020; Unhjem et al., 2016). In addition, between‐muscle effects of ageing on muscle spindles and sensory afferents should be considered when interpreting our data. Although no direct comparison between soleus and tibialis anterior exists, reductions in muscle spindle diameter have been observed in aged deltoid and extensor digitorum, although not in quadriceps or biceps brachii, and decreases in the number of intrafusal fibres in deltoid have also been detected (Kararizou et al., 2005). It is therefore possible that ageing differently affects soleus and tibialis anterior muscle spindles and sensory afferents, and this might be confirmed in future experiments. Interestingly, passive ankle plantar and dorsiflexion detectibly influenced cortico‐spinal (motor evoked potential/maximal compound action potentials) responses in tibialis anterior in young adults but not in older adults, while they remained unchanged in soleus in both groups (Škarabot et al., 2020). These data support the assertion that soleus and tibialis anterior afferent and/or efferent pathways might be differently affected by ageing. Regardless, further direct comparisons between soleus and tibialis anterior are required to elucidate the mechanisms underpinning the dissimilar effects of ageing on PIC inhibitory control between them.
Strengths, limitations and delimitations
Our study employed a validated and extensively used method of PIC strength estimation in humans (Hassan et al., 2021, 2019; Mesquita et al., 2022; Orssatto, Borg et al., 2021; Orssatto, Mackay et al., 2021; ; Trajano et al., 2020; Udina et al., 2010) that shows very good repeated‐measures reliability, as demonstrated by the high reliability of measurements in the control condition of Experiment 1. We used a fifth‐order polynomial function to smooth the instantaneous discharge rate data. This approach has been originally and extensively used to calculate ∆F with the paired‐motor unit analysis technique (Gorassini et al., 2002; Mesquita et al., 2022; Orssatto, Borg et al., 2021; Orssatto, Mackay et al., 2021; Trajano et al., 2020; Udina et al., 2010; Vandenberk & Kalmar, 2014), which allows our ∆F values to be compared with other studies. However, polynomial functions (in particular 5th order) may sometimes over‐smooth the data, resulting in a level of fit error, when compared to the support vector regression approach for example (Beauchamp et al., 2022). Although this would be unlikely to change the interpretation of our results, caution is warranted when comparing the results of studies using different instantaneous discharge rate smoothing methods. Another important limitation is that our findings should not be extrapolated to contraction intensities and motor units with recruitment threshold higher than those used in our study (20% and 30% of peak torques), as discussed previously. Also, our findings relate only to soleus and tibialis anterior as, for example, different muscles have distinct muscle spindle concentrations (Banks, 2006), which may alter the strength of reciprocal inhibition and the effects of ageing. Daily activities require the activation of different muscle groups; thus, future investigations of the effects of ageing on neuromodulatory–inhibitory control of PICs in other muscles, and its influence on possible impairments in physical function, require additional study. Lastly, we have included only non‐sarcopenic older adults, so our results should not be extrapolated to populations with different physical characteristics or potential health conditions, such as the very old (>85 years old), sarcopenic or frail, or those with neurological disorders or individuals with physical activity levels.
Conclusions
We present novel data demonstrating the control of motor neuronal PIC facilitation and inhibition in older adults by estimating PIC strengths using the paired‐motor unit technique. We show that older adults have a preserved ability to amplify PICs through remote muscle contraction (e.g. handgrip, as used presently), which would diffusely increase serotonergic input onto motor neurones in both soleus and tibialis anterior. Subsequently, we present evidence of age‐related reciprocal inhibition‐induced impairment of PIC deactivation in tibialis anterior, which, conversely, was preserved with ageing in soleus. These findings relate to tests completed in mostly lower‐threshold motor units during low intensity contractions (up to 20% or 30% of the individuals’ maximal capacity) and should not be extrapolated to higher threshold motor units or higher intensity contractions. The logical next steps are to explore (i) facilitation–inhibition control of PICs in higher intensity contractions and in different muscles, (ii) the variable control of PICs on motor performance, and (iii) responses to exercise interventions in older adults and other clinical populations.
Additional information
Open research badges
This article has earned an Open Data badge for making publicly available the digitally‐shareable data necessary to reproduce the reported results. The data is available at https://github.com/orssatto/PICs-ageing_2.0.
Competing interests
None.
Author contributions
Conception or design of the work: L.O., A.B., and G.T. Acquisition, analysis or interpretation of data for the work: L.O., G.F., G.T., and A.B. Drafting the work or revising it critically for important intellectual content: L.O., G.F., G.T, and A.B. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The writing of the present manuscript was funded by the Faculty Write‐up scholarship provided by Queensland University of Technology.
Supporting information
Statistical Summary Document
Peer Review History
Supplementary Material 1
Acknowledgements
The authors thank Dr Raphael L. Sakugawa for developing a MATLAB script that facilitated calculation of ΔF, peak discharge rates, and recruitment thresholds.
Open access publishing facilitated by Queensland University of Technology, as part of the Wiley – Queensland University of Technology agreement via the Council of Australian University Librarians.
Biography
Lucas B. R. Orssatto obtained his Bachelor and Master degrees in Physical Education at the Federal University of Santa Catarina (Brazil), and PhD degree at the Queensland University of Technology (Australia). His main research interest is to understand the neurophysiological alterations/adaptations to ageing and exercise. His PhD studies, including the work described herein, explored the effects of ageing and resistance training on intrinsic motor neurone excitability.

Handling Editors: Richard Carson & Mathew Piasecki
The peer review history is available in the Supporting information section of this article (https://doi.org/10.1113/JP283708#support‐information‐section).
[Correction made on 14 November 2022, after first online publication: The article has been updated to include the Open Research Badges section].
This article was first published as a preprint: Orssatto, L. B. R., Fernandes, G. L., Blazevich, A. J., & Trajano, G. S. (2022). Facilitation‐inhibition control of motor neuronal persistent inward currents in young and older adults. bioRxiv, https://doi.org/10.1101/2022.08.08.503135
References
- Aagaard, P. , Suetta, C. , Caserotti, P. , Magnusson, S. P. , & Kjær, M. (2010). Role of the nervous system in sarcopenia and muscle atrophy with aging: Strength training as a countermeasure. Scandinavian Journal of Medicine & Science in Sports, 20, 49–64. [DOI] [PubMed] [Google Scholar]
- Aston‐Jones, G. , Chen, S. , Zhu, Y. , & Oshinsky, M. L. (2001). A neural circuit for circadian regulation of arousal. Nature Neuroscience, 4(7), 732–738. [DOI] [PubMed] [Google Scholar]
- Bakdash, J. Z. , & Marusich, L. R. (2017). Repeated measures correlation. Frontiers in Psychology, 8, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, R. W. (2006). An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. Journal of Anatomy, 208(6), 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp, J. A. , Khurram, O. U. , Dewald, J. P. A. , Heckman, C. J. , & Pearcey, G. E. P. (2022). A computational approach for generating continuous estimates of motor unit discharge rates and visualizing population discharge characteristics. Journal of Neural Engineering, 19(1), 016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, D. , Hagbarth, K.‐E. , Lofstedt, L. , Gunnar Wallin, B. , Martin, C. J. , & Burke, D. (1976). The responses of human muscle spindle endings to vibration of non‐contracting muscles. Journal of Physiology, 261(3), 673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchart, P. , Farquhar, R. , Part, N. , & Roberts, R. (1993). The effect of age and voluntary contraction on presynaptic inhibition of soleus muscle Ia afferent terminals in man. Experimental Physiology, 78(2), 235–242. [DOI] [PubMed] [Google Scholar]
- Cruz‐Jentoft, A. J. , Bahat, G. , Bauer, J. , Boirie, Y. , Bruyère, O. , Cederholm, T. , Cooper, C. , Landi, F. , Rolland, Y. , Sayer, A. A. , Schneider, S. M. , Sieber, C. C. , Topinkova, E. , Vandewoude, M. , Visser, M. , & Zamboni, M. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 (2019). Sarcopenia: Revised European consensus on definition and diagnosis. Age and Ageing, 48(1), 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton, B. H. , McNeil, C. J. , Doherty, T. J. , & Rice, C. L. (2008). Age‐related reductions in the estimated numbers of motor units are minimal in the human soleus. Muscle and Nerve, 38(3), 1108–1115. [DOI] [PubMed] [Google Scholar]
- Frančič, A. , & Holobar, A. (2021). On the reuse of motor unit filters in high density surface electromyograms with different signal‐to‐noise ratios. In Proceedings of the EMBEC 2020, pp. 1–9. IFMBE Proceedings.
- Frančič, A. , & Holobar, A. (2022). Motor unit tracking across low contraction levels of biceps brachii muscle. In Torricelli D., Metin A., & Pons J. L. (eds.), Converging clinical and engineering research on neurorehabilitation IV (pp. 401–405). Springer. Available at: https://www.springer.com/us/book/9783319466682%0A http://link.springer.com/10.1007/978‐3–319‐46669‐9. [Google Scholar]
- Geertsen, S. S. , Willerslev‐Olsen, M. , Lorentzen, J. , & Nielsen, J. B. (2017). Development and aging of human spinal cord circuitries. Journal of Neurophysiology, 118(2), 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini, M. , Yang, J. F. , Siu, M. , & Bennett, D. J. (2002). Intrinsic activation of human motoneurons: Possible contribution to motor unit excitation. Journal of Neurophysiology, 87(4), 1850–1858. [DOI] [PubMed] [Google Scholar]
- Grande, G. , & Cafarelli, E. (2003). Ia afferent input alters the recruitment thresholds and firing rates of single human motor units. Experimental Brain Research, 150(4), 449–457. [DOI] [PubMed] [Google Scholar]
- Hassan, A. S. , Fajardo, M. E. , Cummings, M. , McPherson, L. M. , Negro, F. , Dewald, J. P. A. , Heckman, C. J. , & Pearcey, G. E. (2021). Estimates of persistent inward currents are reduced in upper limb motor units of older adults. Journal of Physiology, 599(21), 4865–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, A. S. , Kim, E. H. , Khurram, O. U. , Cummings, M. , Thompson, C. K. , Miller McPherson, L. , Heckman, C. J. , Dewald, J. P. A. , & Negro, F. (2019). Properties of motor units of elbow and ankle muscles decomposed using high‐density surface EMG. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS 3874–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, A. S. , Thompson, C. K. , Negro, F. , Cummings, M. Q. , Powers, R. K. , Heckman, C. J. , Dewald, J. , & McPherson, L. M. (2020). Impact of parameter selection on estimates of motoneuron excitability using paired motor unit analysis. Journal of Neural Engineering, 17(1), 016063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman, C. J. , Gorassini, M. A. , & Bennett, D. J. (2005). Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle and Nerve, 31(2), 135–156. [DOI] [PubMed] [Google Scholar]
- Heckman, C. J. , Hyngstrom, A. S. , & Johnson, M. D. (2008). Active properties of motoneurone dendrites: Diffuse descending neuromodulation, focused local inhibition. Journal of Physiology, 586(5), 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. , & Baudry, S. (2019). Age‐related changes in leg proprioception: Implications for postural control. Journal of Neurophysiology, 122(2), 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple, R. T. , & Rice, C. L. (2016). Innervation and neuromuscular control in ageing skeletal muscle. Journal of Physiology, 00, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holobar, A. , Minetto, M. A. , & Farina, D. (2014). Accurate identification of motor unit discharge patterns from high‐density surface EMG and validation with a novel signal‐based performance metric. Journal of Neural Engineering, 11(1), 016008. [DOI] [PubMed] [Google Scholar]
- Holobar, A. , & Zazula, D. (2007). Multichannel blind source separation using convolution Kernel compensation. IEEE Transactions on Signal Processing, 55(9), 4487–4496. [Google Scholar]
- Hortobágyi, T. , & Devita, P. (2006). Mechanisms responsible for the age‐associated increase in coactivation of antagonist muscles. Exercise and Sport Sciences Reviews, 34(1), 29–35. [DOI] [PubMed] [Google Scholar]
- Hortobágyi, T. , Del Olmo, M. F. , & Rothwell, J. C. (2006). Age reduces cortical reciprocal inhibition in humans. Experimental Brain Research, 171(3), 322–329. [DOI] [PubMed] [Google Scholar]
- Hortobágyi, T. , Solnik, S. , Gruber, A. , Rider, P. , Steinweg, K. , Helseth, J. , & DeVita, P. (2009). Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait & Posture, 29, 558–564. [DOI] [PubMed] [Google Scholar]
- Hounsgaard, J. , Hultborn, H. , Jespersen, B. , & Kiehn, O. (1988). Bistability of alpha‐motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5‐hydroxytryptophan. Journal of Physiology, 405(1), 345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn, H. , Denton, M. E. , Wienecke, J. , & Nielsen, J. B. (2003). Variable amplication of synaptic input to cat spinal motoneurones by dendritic persistent inward current. Journal of Physiology, 552(3), 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid, L. G. , Aagaard, P. , Ørtenblad, N. , Kjaer, M. , & Suetta, C. (2018). Plasticity in central neural drive with short‐term disuse and recovery ‐ effects on muscle strength and influence of aging. Experimental Gerontology, 106, 145–153. [DOI] [PubMed] [Google Scholar]
- Hyngstrom, A. S. , Johnson, M. D. , Miller, J. F. , & Heckman, C. J. (2007). Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nature Neuroscience, 10(3), 363–369. [DOI] [PubMed] [Google Scholar]
- Jacobs, B. L. , Martín‐Cora, F. J. , & Fornal, C. A. (2002). Activity of medullary serotonergic neurons in freely moving animals. Brain Research Reviews, 40(1–3), 45–52. [DOI] [PubMed] [Google Scholar]
- Johnson, H. , Ulfhake, B. , Dagerlind, Å. , Bennett, G. W. , Fone, K. C. F. , & Hökfelt, T. (1993). The serotoninergic bulbospinal system and brainstern‐spinal cord content of serotonin‐, TRH‐, and substance P‐like immunoreactivity in the aged rat with special reference to the spinal cord motor nucleus. Synapse, 15(1), 63–89. [DOI] [PubMed] [Google Scholar]
- Kararizou, E. , Manta, P. , Kalfakis, N. , & Vassilopoulos, D. (2005). Morphometric study of the human muscle spindle. Analytical and Quantitative Cytology and Histology, 27, 1–4. [PubMed] [Google Scholar]
- Kido, A. , Tanaka, N. , & Stein, R. B. (2004). Spinal excitation and inhibition decrease as humans age. Canadian Journal of Physiology and Pharmacology, 82(4), 238–248. [DOI] [PubMed] [Google Scholar]
- Klass, M. , Baudry, S. , & Duchateau, J. (2011). Modulation of reflex responses in activated ankle dorsiflexors differs in healthy young and elderly subjects. European Journal of Applied Physiology, 111(8), 1909–1916. [DOI] [PubMed] [Google Scholar]
- Ko, M. L. , King, M. A. , Gordon, T. L. , & Crisp, T. (1997). The effects of aging on spinal neurochemistry in the rat. Brain Research Bulletin, 42(2), 95–98. [DOI] [PubMed] [Google Scholar]
- Kuo, J. J. , Lee, R. H. , Johnson, M. D. , Heckman, H. M. , & Heckman, C. J. (2003). Active dendritic integration of inhibitory synaptic inputs in vivo. Journal of Neurophysiology, 90(6), 3617–3624. [DOI] [PubMed] [Google Scholar]
- Kurz, E. , Faude, O. , Roth, R. , Zahner, L. , & Donath, L. (2018). Ankle muscle activity modulation during single‐leg stance differs between children, young adults and seniors. European Journal of Applied Physiology, 118(2), 239–247. [DOI] [PubMed] [Google Scholar]
- Kuznetsova, A. , Brockhoff, P. B. , & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. [Google Scholar]
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t‐tests and ANOVAs. Frontiers in Psychology, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughton, C. A. , Slavin, M. , Katdare, K. , Nolan, L. , Bean, J. F. , Kerrigan, D. C. , Phillips, E. , Lipsitz, L. A. , & Collins, J. J. (2003). Aging, muscle activity, and balance control: Physiologic changes associated with balance impairment. Gait & Posture, 18, 101–108. [DOI] [PubMed] [Google Scholar]
- Lee, R. H. , & Heckman, C. J. (1999). Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic α1 agonist methoxamine. Journal of Neurophysiology, 81(5), 2164–2174. [DOI] [PubMed] [Google Scholar]
- Lee, R. H. , & Heckman, C. J. (2000). Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. Journal of Neuroscience, 20(17), 6734–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth, R. V. , Buerkner, P. , Herve, M. , Love, J. , Riebl, H. , & Singmann, H. (2021). Emmeans: Estimated marginal means, aka least‐squares means – R package. Available at: https://github.com/rvlenth/emmeans.
- Liu, K. Y. , Kievit, R. A. , Tsvetanov, K. A. , Betts, M. J. , Düzel, E. , Rowe, J. B. , Cam‐CAN, Howard, R. , & Hämmerer D (2020). Noradrenergic‐dependent functions are associated with age‐related locus coeruleus signal intensity differences. Nature Communications, 11, 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso, A. , Nimmo, M. A. , Foster, J. E. , Cockburn, M. , McMillan, N. C. , & de Vito, G. (2002). Contractile muscle volume and agonist‐antagonist coactivation account for differences.pdf. Muscle & Nerve, 25, 858–863. [DOI] [PubMed] [Google Scholar]
- Manini, T. M. , Hong, S. L. , & Clark, B. C. (2013). Aging and muscle: a neuron's perspective. Current Opinion in Clinical Nutrition and Metabolic Care, 16(1), 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masani, K. , Sayenko, D. G. , & Vette, A. H. (2013). What triggers the continuous muscle activity during upright standing? Gait & Posture, 37, 72–77. [DOI] [PubMed] [Google Scholar]
- McGregor, K. M. , Zlatar, Z. , Kleim, E. , Sudhyadhom, A. , Bauer, A. , Phan, S. , Seeds, L. , Ford, A. , Manini, T. M. , White, K. D. , Kleim, J. , & Crosson, B. (2011). Physical activity and neural correlates of aging: A combined TMS /fMRI study. Behavioural Brain Research, 222(1), 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita, R. N. O. , Taylor, J. L. , Trajano, G. S. , Škarabot, J. , Holobar, A. , Gonçalves, B. A. M. , & Blazevich, A. J. (2022). Effects of reciprocal inhibition and whole‐body relaxation on persistent inward currents estimated by two different methods. Journal of Physiology, 600(11), 2765–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Míguez, J. M. , Aldegunde, M. , Paz‐Valiñas, L. , Recio, J. , & Sánchez‐Barceló, E. (1999). Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging. Journal of Neural Transmission, 106, 1089–1098. [DOI] [PubMed] [Google Scholar]
- de Oliveira, D. S. , Casolo, A. , Balshaw, T. G. , Maeo, S. , Lanza, M. B. , Martin, N. R. W. , Maffulli, N. , Kinfe, T. M. , Eskofier, B. , Folland, J. P. , Farina, D. , & Del Vecchio, A. (2022). Neural decoding from surface high‐density EMG signals: Influence of anatomy and synchronization on the number of identified motor units. Journal of Neural Engineering, 19(4), 046029. 10.1088/1741-2552/ac823d [DOI] [PubMed] [Google Scholar]
- Orssatto, L. B. R. , Wiest, M. J. , & Diefenthaeler, F. (2018). Neural and musculotendinous mechanisms underpinning age‐related force reductions. Mechanisms of Ageing and Development, 175, 17–23. [DOI] [PubMed] [Google Scholar]
- Orssatto, L. B. R. , Borg, D. N. , Blazevich, A. J. , Sakugawa, R. L. , Shield, A. J. , & Trajano, G. S. (2021). Intrinsic motoneuron excitability is reduced in soleus and tibialis anterior of older adults. GeroScience, 43(6), 2719–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orssatto, L. B. R. , Borg, D. N. , Pendrith, L. , Blazevich, A. J. , Shield, A. J. , & Trajano, G. S. (2022). Do motoneuron discharge rates slow with aging? A systematic review and meta‐analysis. Mechanisms of Ageing and Development, 203, 111647. [DOI] [PubMed] [Google Scholar]
- Orssatto, L. B. R. , Mackay, K. , Shield, A. J. , Sakugawa, R. L. , Blazevich, A. J. , & Trajano, G. S. (2021). Estimates of persistent inward currents increase with the level of voluntary drive in low‐threshold motor units of plantar flexor muscles. Journal of Neurophysiology, 125(5), 1746–1754. [DOI] [PubMed] [Google Scholar]
- Orssatto, L. B. R. , Rodrigues, P. , Philips, K. M. , Blazevich, A. J. , Borg, D. N. , de Souza, T. R. , Sakugawa, R. L. , Shield, A. J. , & Trajano, G. S. (2022). Intrinsic motor neurone excitability is increased after resistance training in older adults Lucas. SportRxiv, 10.51224/SRXIV.144 [DOI] [PubMed] [Google Scholar]
- Orssatto, L. B. R. , Wiest, M. J. , Moura, B. M. , Collins, D. F. , & Diefenthaeler, F. (2020). Neuromuscular determinants of explosive torque: Differences among strength‐trained and untrained young and older men. Scandinavian Journal of Medicine & Science in Sports, 30(11), 2092–2100. [DOI] [PubMed] [Google Scholar]
- Pearcey, G. E. P. , Khurram, O. U. , Beauchamp, J. A. , Negro, F. , & Heckman, C. J. (2022). Antagonist tendon vibration dampens estimates of persistent inward currents in motor units of the human lower limb. bioRxiv, 10.1101/2022.08.02.502526 [DOI] [Google Scholar]
- Polcyn, A. E. , Kerrigan, D. C. , & Collins, J. J. (1998). Age‐related changes in the initiation of gait: Degradation of central mechanisms for momentum generation. Archives of Physical Medicine and Rehabilitation, 79(12), 1582–1589. [DOI] [PubMed] [Google Scholar]
- Revill, A. L. , & Fuglevand, A. J. (2017). Inhibition linearizes firing rate responses in human motor units: Implications for the role of persistent inward currents. Journal of Physiology, 595(1), 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglioni, G. , Ferri, A. , Minetti, A. E. , Martin, A. , Hoecke, J. , & Narici, M. V. (2012). Plantar flexor activation capacity and H reflex in older adults : adaptations to strength training. Journal of Applied Physiology, 92(6), 2292–2302. [DOI] [PubMed] [Google Scholar]
- Scaglioni, G. , Narici, M. V. , Maffiuletti, N. A. , Pensini, M. , & Martin, A. (2003). Effect of ageing on the electrical and mechanical properties of human soleus motor units activated by the H reflex and M wave. Journal of Physiology, 548(2), 649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, E. , Sasaki, M. , Tohyama, K. , Kanbara, Y. , Otsuka, K. , Ehara, S. , & Sakai, A. (2006). Age‐related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magnetic Resonance in Medical Sciences, 5, 197–200. [DOI] [PubMed] [Google Scholar]
- Škarabot, J. , Ansdell, P. , Brownstein, C. G. , Hicks, K. M. , Howatson, G. , Goodall, S. , & Durbaba, R. (2019). Reduced corticospinal responses in older compared with younger adults during submaximal isometric, shortening, and lengthening contractions. Journal of Applied Physiology, 126(4), 1015–1031. [DOI] [PubMed] [Google Scholar]
- Škarabot, J. , Ansdell, P. , Howatson, G. , Goodall, S. , & Durbaba, R. (2020). Corticospinal responses during passive shortening and lengthening of tibialis anterior and soleus in older compared to younger adults. Experimental Physiology, 105(3), 419–426. [DOI] [PubMed] [Google Scholar]
- Soames, R. W. , & Atha, J. (1981). The role of the antigravity musculature during quiet standing in man. European Journal of Applied Physiology and Occupational Physiology, 47(2), 159–167. [DOI] [PubMed] [Google Scholar]
- Steinbusch, H. W. M. , Dolatkhah, M. A. , & Hopkins, D. A. (2021). Anatomical and neurochemical organization of the serotonergic system in the mammalian brain and in particular the involvement of the dorsal raphe nucleus in relation to neurological diseases. In Progress in Brain Research, 261, 41–81. [DOI] [PubMed] [Google Scholar]
- Suetta, C. , Haddock, B. , Alcazar, J. , Noerst, T. , Hansen, O. M. , Ludvig, H. , Kamper, R. S. , Schnohr, P. , Prescott, E. , Andersen, L. L. , Frandsen, U. , Aagaard, P. , Bülow, J. , Hovind, P. , & Simonsen, L. (2019). The copenhagen sarcopenia study: lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. Journal of Cachexia, Sarcopenia and Muscle, 10(6), 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow, M. (1966). Fibre size and content of the anterior tibial nerve of the foot. Journal of Neurology, Neurosurgery, and Psychiatry, 29(3), 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajano, G. S. , Taylor, J. L. , Orssatto, L. B. R. , McNulty, C. R. , & Blazevich, A. J. (2020). Passive muscle stretching reduces estimates of persistent inward current strength in soleus motor units. Journal of Experimental Biology, 223, jeb.229922. [DOI] [PubMed] [Google Scholar]
- Udina, E. , D'Amico, J. , Bergquist, A. J. , & Gorassini, M. A. (2010). Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor‐unit activity. Journal of Neurophysiology, 103(3), 1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unhjem, R. , Nygård, M. , van den Hoven, L. T. , Sidhu, S. K. , Hoff, J. , & Wang, E. (2016). Lifelong strength training mitigates the age‐related decline in efferent drive. Journal of Applied Physiology, 121(2), 415–423. [DOI] [PubMed] [Google Scholar]
- Vandenberk, M. S. , & Kalmar, J. M. (2014). An evaluation of paired motor unit estimates of persistent inward current in human motoneurons. Journal of Neurophysiology, 111(9), 1877–1884. [DOI] [PubMed] [Google Scholar]
- Vaughan, S. K. , Stanley, O. L. , & Valdez, G. (2017). Impact of aging on proprioceptive sensory neurons and intrafusal muscle fibers in mice. Journals of Gerontology Series A, Biological Sciences and Medical Sciences, 72, 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, K. , Glaser, J. I. , Deng, L. , Thompson, C. K. , Stevenson, I. H. , Wang, Q. , Hornby, T. G. , Heckman, C. J. , & Kording, K. P. (2014). Serotonin affects movement gain control in the spinal cord. Journal of Neuroscience, 34(38), 12690–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Z. , Guindani, M. , Grieco, S. F. , Chen, L. , Holmes, T. C. , & Xu, X. (2021). Beyond t test and ANOVA: applications of mixed‐effects models for more rigorous statistical analysis in neuroscience research. Neuron, 110(1), 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document
Peer Review History
Supplementary Material 1


