Figure 3.
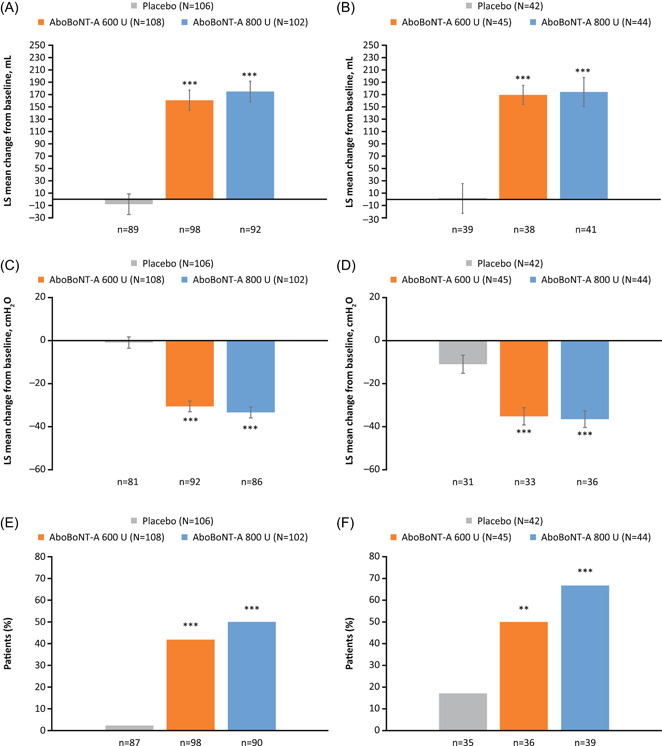
Change from baseline in MCC and MDFP,a and proportion of patients with no IDCb at Week 6 in patients with SCI (A, C, E) and MS (B, D, F). **p < 0.01; ***p < 0.001 vs. placebo. Data for (A)–(D) are LS mean ± SE. Data shown are for urodynamic pooled population (DBPC period). aBased on an analysis of covariance model to assess difference in change from baseline between each aboBoNT‐A dose group (600 or 800 U) versus placebo at Week 6, with treatment group, stratification factors (etiology of NDOI [SCI or MS], prior intradetrusor use [BoNT‐A naïve or non‐naïve]), study baseline value, study and the treatment‐by‐etiology group interaction as fixed effect variables; bBased on logistic regression model to assess difference between each aboBoNT‐A dose group (600 or 800 U) versus placebo at Week 6, with treatment group, stratification factors (etiology of NDOI [SCI or MS], prior intradetrusor use [BoNT‐A naïve or non‐naïve]), study, and treatment‐by‐etiology group interaction as fixed variables. AboBoNT‐A, abobotulinumtoxinA; BoNT‐A, botulinum toxin type A; DBPC, double‐blind placebo‐controlled; IDC, involuntary detrusor contraction; LS, least squares; MCC, maximum cystometric capacity; MDFP, maximum detrusor filling pressure; MS, multiple sclerosis; N, number of patients in treatment group; n, number of evaluable patients; NDOI, neurogenic detrusor overactivity incontinence; SCI; spinal cord injury; SE, standard error; U, units
