FIGURE 1.
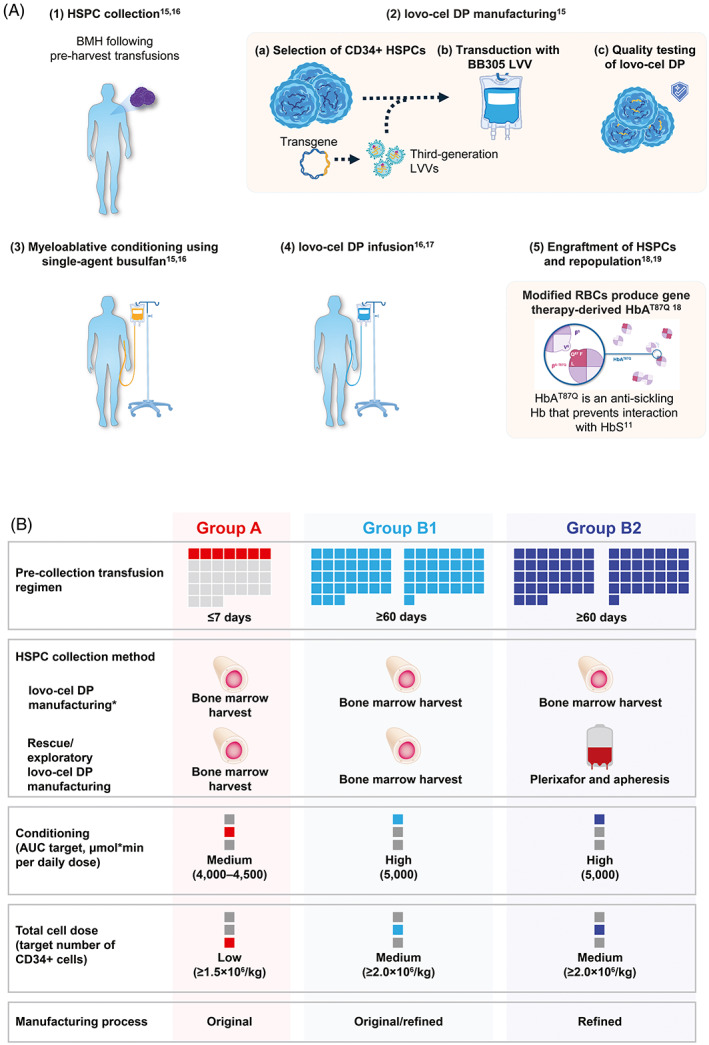
Overview of the lovo‐cel treatment process and HGB‐206 study design evolution. (A) Gene therapy treatment process of lovo‐cel for SCD. (B) Treatment process evolution. *The target TNC for BMH was ≥6 × 108/kg per patient. The Group B1 patient received lovo‐cel produced using both the original and refined manufacturing process, and the Group B2 patient received lovo‐cel produced using only the refined manufacturing process. Both Group B patients received lovo‐cel manufactured from HSPCs collected using BMH; however, the Group B2 patient also underwent rescue HSPC collection by plerixafor mobilization/apheresis for the exploratory evaluation of its safety and enhancement of the lovo‐cel manufacturing process. The Group B2 patient did not receive lovo‐cel manufactured from HSPCs collected using plerixafor mobilization/apheresis. Figure adapted from N Engl J Med, Kanter, J. et al., Biologic and Clinical Efficacy of LentiGlobin for Sickle Cell Disease, 386, 617–628. Copyright © 2022 Massachusetts Medical Society. Reprinted with permission. 16 AUC, area under the curve; BMH, bone marrow harvest; DP, drug product; HbAT87Q, Hb with modified β‐globin gene (βA‐T87Q); HbS, sickle Hb; HSPC, hematopoietic stem and progenitor cell; LVV, lentiviral vector; RBC, red blood cell; SCD, sickle cell disease; TNC, total nucleated cell count. [Color figure can be viewed at wileyonlinelibrary.com]
