Abstract
Objective
Our aim was to assess the real‐world effectiveness of immune checkpoint inhibitors for treatment of patients with progressive multifocal leukoencephalopathy (PML).
Methods
We conducted a multicenter survey compiling retrospective data from 79 PML patients, including 38 published cases and 41 unpublished cases, who received immune checkpoint inhibitors as add‐on to standard of care. One‐year follow‐up data were analyzed to determine clinical outcomes and safety profile. Logistic regression was used to identify variables associated with 1‐year survival.
Results
Predisposing conditions included hematological malignancy (n = 38, 48.1%), primary immunodeficiency (n = 14, 17.7%), human immunodeficiency virus/acquired immunodeficiency syndrome (n = 12, 15.2%), inflammatory disease (n = 8, 10.1%), neoplasm (n = 5, 6.3%), and transplantation (n = 2, 2.5%). Pembrolizumab was most commonly used (n = 53, 67.1%). One‐year survival was 51.9% (41/79). PML–immune reconstitution inflammatory syndrome (IRIS) was reported in 15 of 79 patients (19%). Pretreatment expression of programmed cell death‐1 on circulating T cells did not differ between survivors and nonsurvivors. Development of contrast enhancement on follow‐up magnetic resonance imaging at least once during follow‐up (OR = 3.16, 95% confidence interval = 1.20–8.72, p = 0.02) was associated with 1‐year survival. Cerebrospinal fluid JC polyomavirus DNA load decreased significantly by 1‐month follow‐up in survivors compared to nonsurvivors (p < 0.0001). Thirty‐two adverse events occurred among 24 of 79 patients (30.4%), and led to treatment discontinuation in 7 of 24 patients (29.1%).
Interpretation
In this noncontrolled retrospective study of patients with PML who were treated with immune checkpoint inhibitors, mortality remains high. Development of inflammatory features or overt PML‐IRIS was commonly observed. This study highlights that use of immune checkpoint inhibitors should be strictly personalized toward characteristics of the individual PML patient. ANN NEUROL 2023;93:257–270
Progressive multifocal leukoencephalopathy (PML) is a rare but devastating opportunistic infection of the central nervous system due to the reactivation of JC polyomavirus (JCV) in immunocompromised patients. 1 Because there is no effective antiviral treatment against JCV, recovery of antiviral immune responses remains the only available treatment of PML. Emerging therapeutic strategies aimed at promoting this recovery have received increasing attention, including the use of cytokines such as interleukin‐7 (IL‐7), 2 adoptive transfer of anti‐polyomavirus specific T cells,3 and immune checkpoint inhibitors (ICIs). 4 , 5
Inhibitory immune checkpoint molecules such as programmed cell death‐1 (PD‐1) are receptors expressed by activated T cells that play a vital role in the maintenance of peripheral tolerance. 6 Experimental evidence suggests that upregulation of PD‐1 by T cells, and of its ligands PD‐L1 and PD‐L2 by hematopoietic and many nonhematopoietic cell types, during acute viral infection protects surrounding tissues from excessive primary T‐cell responses. 7 However, in the setting of chronic antigen stimulation, such as during cancer or chronic infection, persistent upregulated expression of PD‐1 and other immune checkpoint molecules triggers inhibitory signaling pathways that mediate a complex state of T‐cell dysfunction named exhaustion, resulting in tumor or viral immune evasion. 8 ICIs, by reversing T‐cell exhaustion, have been proposed as a strategy to reinvigorate antiviral activity in chronic infections such as PML. 9 , 10
As of September 1, 2021, 49 patients who received ICIs for treatment of PML were reported in the literature in isolated case reports and small case series (Supplementary References), most of them suggesting clinical benefit that may represent publication bias in favor of positive results, a well‐known occurrence in the field of PML. To get around this risk, we conducted a large multicenter survey to retrospectively compile both published and unpublished cases of patients who received ICIs as add‐on to standard of care for PML. Our primary outcome was survival at 1 year after ICI initiation. Survival of a patient with PML hinges on several key requirements: (1) sufficient activation of antiviral immune responses, which must be kept in check so as not to contribute to morbidity or even mortality due to immune reconstitution inflammatory syndrome (IRIS) 11 ; (2) sustained control of JCV replication, which must be achieved before brain injury of vital structures is experienced; and (3) adequate control of the underlying disease, which can reactivate in the setting of discontinuation of treatments that predispose to the development of PML. Therefore, secondary outcomes in this study included estimated proportion of patients who experienced control of JCV replication or who developed contrast‐enhancing lesions on brain magnetic resonance imaging (MRI) and/or PML‐IRIS, both considered indirect measures of ICI biological effect, and specific cause of death as attributed by the treating physician. Lastly, we explored factors associated with survival at 1 year and overall safety profile of ICIs.
Patients and Methods
Study Design and Participants
Published case reports or case series as of September 1, 2021 were identified via literature search of PubMed and Web of Science using the keywords “progressive multifocal leukoencephalopathy”, “JC virus”, “immune checkpoint inhibitors”, and “PD‐1.”. Authors were invited to participate in this retrospective survey. Unpublished cases, regardless of underlying predisposing condition or outcome of PML, were solicited from the same authors and their networks, as well as through the mailing lists of French national networks of infectious diseases physicians (Société de Pathologie Infectieuse de Langue Française), neurologists (Société Française de Neurologie, Observatoire Français de la SEP), and internists (Société Nationale Française de Médecine Interne).
Adults (age ≥ 18 years) having received at least one infusion of anti‐PD‐1 or anti‐PD‐L1 monoclonal antibodies as of December 2020 for treatment of definite PML (defined according to the 2013 consensus diagnostic criteria for PML as compatible clinical and imaging findings and either JCV DNA detected in cerebrospinal fluid [CSF] or confirmatory histological analysis by brain biopsy 12 ) were included. Patients missing PML outcome at 12 months after ICI initiation (including published cases for whom the authors did not reply to invitation to submit additional data) and patients who received other immunotherapies concomitantly or during the 12‐month period before or after ICI administration were excluded.
This study was approved by the institutional review board of the Toulouse University Hospital (RnIPH 2020–95), in accordance with the French data protection authority (MR004, Commission Nationale de l'Informatique et des Libertés, CNIL number 2206723v0).
Data Collection and Endpoints
Data for this study were retrospectively collected using an anonymized case report form (CRF). The CRF collected demographic data and details of clinical and treatment history of the predisposing disease and of the PML, including details of neurological examination and modified Rankin Scale (mRS). Administration schedules of ICIs were in accordance with local practice. Results of diagnostic testing including CSF JCV polymerase chain reaction (PCR), contrast‐enhanced MRI of the brain, and brain biopsy and results of peripheral blood immunophenotyping, including total lymphocyte and subpopulations counts and analysis of PD‐1 expression, were also collected, when performed. CSF JCV PCR was performed according to local methods, and quantitative results were expressed as log copies/ml. Analysis of percentage of circulating CD4+ and CD8+ whole T‐cell populations expressing PD‐1 was performed by multiparametric flow cytometry according to local protocols. No imputation of missing data was performed. Four causes of death were considered, determined by the treating physician: death due to PML progression, death due to PML‐IRIS, death due to progression of both PML and the PML‐related underlying condition, or death due to progression of the PML‐related underlying condition. Diagnosis of PML‐IRIS was defined as clinical worsening with an increase by at least 1 point on the mRS, together with evidence of new or worsening contrast‐enhancing lesions and/or edema detected by brain MRI. 1 Adverse events were collected for all patients, but not graded.
The primary endpoint was the proportion of patients surviving 12 months after initiation of treatment with ICIs. Secondary endpoints were (1) proportion of patients with a decrease in CSF JCV DNA load of >1 log copies/ml at least once during follow‐up, (2) proportion of patients having contrast‐enhancing lesions on brain MRI at least once during follow‐up and/or developing PML‐IRIS, and (3) specific cause of death.
Statistical Analyses
Descriptive analysis was performed using median with interquartile range (IQ25–75) for quantitative variables, and counts and proportions for qualitative data. Logistic regression was used to analyze baseline variables associated with 12‐month survival after the first ICI infusion (yes/no). Explanatory variables collected at baseline were sex, age at PML diagnosis, underlying predisposing condition, history and duration of immunosuppressive treatment before PML diagnosis and its continuation afterward, time between first symptoms, PML diagnosis, and ICI initiation, mRS, presence of contrast‐enhancing lesions, number of brain regions involved, blood absolute lymphocyte, CD4+ T‐cell, and CD8+ T‐cell counts at baseline (/μl), and type of ICI. Univariate models contained the response variable and one of these variables. A multivariate model was constructed including all explanatory variables with a p value <0.20 on the univariate analysis. Lymphocyte counts, CD4+ T‐cell counts, and CD8+ T‐cell counts were measured at baseline and at first follow‐up after ICI initiation. The proportion of patients with an increase of >50% between baseline and first follow‐up was calculated, and compared using a logistic regression for the CD4+ T‐cell counts, and using a Fisher exact test for the lymphocyte counts and the CD8+ T‐cell counts, to compare survivors and nonsurvivors. Expression of PD‐1 by circulating CD4+ T cells and CD8+ T cells and CSF JCV DNA were measured and compared at baseline between survivors and nonsurvivors using a logistic regression. The same 3 variables were also measured and compared at first follow‐up after ICI initiation between survivors and nonsurvivors using a logistic regression. For the expression of PD‐1 by circulating CD4+ T cells and CD8+ T cells, we calculated the number of patients with a percentage of <5% during the first follow‐up, and then compared survivors and nonsurvivors using a Fisher exact test. For the CSF JCV DNA, we calculated the number of patients with a decrease of >1 log from baseline at first monitoring, and then compared survivors and nonsurvivors using a logistic regression. Survival analysis using Kaplan–Meier curves was used to describe survival over 1 year following ICI. All tests were 2‐sided and considered significant at an α level of 0.05. Statistical analyses were conducted using R version 4.0.2.
Results
Patient Characteristics and Management of PML
Survey data were received for a total of 91 patients with proven PML who received ICIs during the study period. Of these, 10 who received additional immunotherapies (3 patients received interleukin‐2, 3 recombinant human IL‐7, 2 viral‐specific T cells, 1 interferon gamma, and 1 granulocyte‐colony stimulating factor), and 2 published cases having missing outcome at 12 months (1 patient living with human immunodeficiency virus [HIV]/acquired immunodeficiency syndrome [AIDS] and one with follicular lymphoma) were excluded. Seventy‐nine patients were included (87%), coming from 9 countries (France [30 patients from 13 centers], the USA [15 patients from 5 centers], Germany [14 patients from 7 centers], Switzerland [8 patients from 3 centers], Italy [6 patients from a single center], the Netherlands [2 patients from a single center], Belgium [2 patients from a single center], Taiwan, and Israel [1 patient each]). Among these, 38 of 79 (48.1%) were previously published cases (Supplementary References), and 41 of 79 (51.9%) were unpublished. Baseline characteristics before initiation of ICI are presented in Table 1. Diagnosis of PML was based on histopathology in 5 patients (6.3%), detection of JCV DNA in CSF in 56 patients (70.9%), and both in 18 patients (22.8%).
TABLE 1.
Main Characteristics of 79 PML Patients at ICI Initiation
| Characteristic | All, n = 79 | Hematological Malignancies, n = 38 a | Primary Immunodeficiencies, n = 14 b | HIV/AIDS, n = 12 | Chronic Inflammatory Diseases, n = 8 c | Solid Neoplasm, n = 5 d | Transplant Recipients, n = 2 e |
|---|---|---|---|---|---|---|---|
| Male sex, n (%) | 52 (65.8) | 24 (63.1) | 10 (71.4) | 8 (66.7) | 4 (50) | 5 (100) | 1 (50) |
| Age at PML diagnosis, yr, median (IQ25–75) | 62.0 (51.0–71.5) | 69.5 (61.8–75.8) | 50.0 (42.8–59.5) | 47.5 (35.5–51.5) | 61.0 (57.5–71.25) | 70.0 (66.0–71.0) | 74.0 (70.5–77.5.) |
| Time from symptom onset to PML diagnosis, days, median (IQ25–75) |
39 (19.0–73.5) [78/79] |
45 (19.0–68.0) [37/38] |
47.5 (33.3–77.8) | 37 (11.5–134.3) | 33.5 (27.5–67.3) | 19 (14–53) | 21.5 (16.3–26.8) |
| Time from PML diagnosis to ICI initiation, days, median (IQ25–75) | 22.5 (10.3–39.5) [78/79] | 19 (6–35) [37/38] | 26 (19.8–61.0) | 27.5 (20.8–36.5) | 31.5 (12.3–49.0) | 22 (19–43) | 8.5 (7.3–9.8) |
| mRS, median (IQ25–75) | 3 (2–4) [76/79] | 3 (3–4) [36/38] | 3 (3–4) | 3 (2–4) | 4 (3–4) [7/8] | 3 (2–4) | 5 (5–5) |
| Brain lobes affected, n, median (IQ25–75) | 3 (2–4) | 3 (2–5) | 3 (2–3) | 4 (3–5) | 3 (2–3) | 3 (2–4) | 3 (3–4) |
| Patients with PML contrast‐enhancing lesions, n (%) | 21 (26.6) | 10 (26.3) | 2 (14.3) | 3 (25) | 4 (50) | 2 (40) | 0 (0) |
| Blood lymphocyte count per μl, median (IQ25–75) | 860 (555–1,286.5) [70/79] | 850 (560–1,335.5) [31/38] | 790 (580–879) | 1,308.5 (1,228.3–2,015) | 675 (490–950) [6/8] | 870 (530–1,270) | 331.5 (253.8–409.3) |
| Blood CD4+ T‐cell count per μl, median (IQ25–75) | 185 (107.5–354.5) [70/79] | 196 (109–418.5) [31/38] | 205.5 (102.3–346.3) | 119 (84.3–249) | 203 (158.3–277.8) [6/8] | 154 (140–217) | 181.5 (128.8–234.3) |
| Blood CD8+ T‐cell count per μl, median (IQ25–75) | 273.5 (84.5–584.8) [68/79] | 235 (91.3–373.8) [30/38] | 184.5 (75–497.3) | 853.5 (616–1,036.3) | 203.5 (77.5–330.3) [6/8] | 164.5 (105.3–257.5) [4/5] | 63 (61–65) |
| JCV DNA level in CSF, log copies/ml, median (IQ25–75) f | 3.8 (2.7–4.9) [60/79] | 4.16 (2.7–5.3) [26/38] | 3.7 (2.8–4.5) [12/14] | 4.0 (2.8–4.4) [11/12] | 4.4 (3.2–5.4) [6/8] | 2.8 (2.4–3.5) [4/5] | 2.9 (2.9–2.9) [1/2] |
| Immunosuppressive treatment before PML diagnosis, n (%) g | 47 (59.5) | 36 (94.7) | 2 (14.3) | 1 (8.3) h | 6 (75) | 0 (0) | 2 (100) |
All baseline variables refer to data at or closest to the time of ICI initiation, unless specified.
In cases of missing data, the actual number of patients is indicated in brackets after each result.
B‐cell non‐Hodgkin lymphoma (23/38, 60.5%), chronic lymphoid leukemia (8/38, 21.1%), Hodgkin lymphoma (3/38, 7.9%), T‐cell lymphoma (2/38, 5.3%), myeloma (1/38), and acute myeloid leukemia (1/38).
Primary immunodeficiencies included idiopathic common variable immunodeficiency (n = 11, 1 defect in DOCK8, 1 defect in CD40 ligand, others without an identified genetic defect), idiopathic CD4 T‐cell lymphocytopenia (n = 2), idiopathic CD8 T‐cell lymphocytopenia (n = 1).
Multiple sclerosis (n = 2), sarcoidosis (n = 1), rheumatoid arthritis (n = 1), myasthenia gravis (n = 1), Erdheim–Chester disease (n = 1), systemic lupus erythematosus (n = 1), overlap syndrome (n = 1). Seven received immunosuppressive drugs; the patient with Erdheim–Chester disease received interferon‐alpha.
Lung cancer (n = 2), prostate cancer (n = 1), cholangiocarcinoma (n = 1), hepatocellular carcinoma (n = 1). None had chemotherapy before PML onset, but 1 had radiotherapy for prostate cancer, and 1 had hepatic embolization for cholangiocarcinoma.
Kidney transplant (n = 2).
Among the 71 patients with CSF JCV DNA positivity at diagnosis, 11 patients (15.5%) had qualitative results only.
Immunosuppressive medications were represented by chemotherapy with or without rituximab or ibrutinib for 35/47 patients (74.5%, including 1 allogeneic hematopoietic stem cell transplantation), rituximab only for 5 patients (10.7%), fingolimod only for 1 patient (1.8%), steroids, methotrexate, or leflunomide for 3 patients (10.6%), and antirejection therapies for 2 patients (4.3%).
This HIV/AIDS patient received etoposide and rituximab for a multicentric Castleman disease.
Abbreviation: AIDS = acquired immunodeficiency syndrome; CSF = cerebrospinal fluid; HIV = human immunodeficiency virus; ICI = immune checkpoint inhibitor; IQ25–75 = interquartile range; JCV = JC polyomavirus; mRS = modified Rankin Scale; PML = progressive multifocal leukoencephalopathy.
Forty‐seven patients (59.5%) had received treatment with immunosuppressive medications prior to PML diagnosis. After a median exposure of 442 days (IQ25–75 = 121–1,240, 9 missing data), immune suppressive medications were discontinued in 39 of 47 patients. Although the median time between treatment discontinuation and diagnosis of PML was 53 days (IQ25–75 = 35–191), this is influenced by the predominance of patients with hematological malignancies, representing 32 of 39 (82%), who had completed chemotherapy courses prior to onset of PML symptoms. None of the 5 patients with solid neoplasm had received systemic chemotherapy before PML diagnosis. Among patients living with HIV/AIDS, 6 of 12 (50%) developed PML while stable on antiretroviral therapy (ART) with control of HIV viremia, whereas 6 of 12 (50%) had undergone ART initiation or optimization with a median time before first ICI infusion of 47 days (IQ25–75 = 24–101). All received optimized ART following PML diagnosis with control of HIV replication.
ICI treatment regimens are described in Table 2. The most commonly used ICI was pembrolizumab (2mg/kg, n = 53, 67.1%). Nivolumab was administered in 24 patients (30.4%), at a dosage of 3mg/kg. The 2 remaining patients were treated with atezolizumab (1 received 3 injections at 20mg/kg, the other 10 injections at 14mg/kg).
TABLE 2.
PML Treatment
| Treatment | All, n = 79 | Hematological Malignancies, n = 38 | Primary Immunodeficiencies, n = 14 | HIV/AIDS, n = 12 | Chronic Inflammatory Diseases, n = 8 | Solid Neoplasm, n = 5 | Transplant Recipients, n = 2 |
|---|---|---|---|---|---|---|---|
| Type of ICI, n (%) | |||||||
| Nivolumab | 24 (30.4) | 9 (23.7) | 6 (42.9) | 5 (41.7) | 1 (12.5) | 1 (20.0) | 2 (100) |
| Pembrolizumab | 53 (67.1) | 28 (73.7) | 8 (57.1) | 7 (58.3) | 7 (87.5) | 3 (60) | 0 (0) |
| Atezolizumab | 2 (2.5) | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) |
| ICI injections, n, median (IQ25–75) | |||||||
| All | 3 (2–4) | 3 (2–4) | 3.5 (3–12.5) | 2 (1.8–2.5) | 2.5 (1.8–3.3) | 2 (1–2) | 2 (2–2) |
| Nivolumab | 3 (2–4.3) | 3 (2–3) | 15 (7.5–21) | 2 (1.5–2.5) | 2 (2–2) | 2 (2–2) | 2 (2–2) |
| Pembrolizumab | 3 (2–4) | 3 (2–4.3) | 3 (2–3.3) | 2 (2–4) | 3 (1.5–3.5) | 1 (1–1.5) | — |
| Atezolizumab | 6.5 (4.8–8.3) | 3 | — | — | — | 10 | — |
| Other treatments, n (%) | |||||||
| Mirtazapine | 25 (31.7) | 7 (18.4) | 5 (35.7) | 8 (66.7) | 2 (25) | 1 (20) | 2 (100) |
| Mefloquine | 6 (7.6) | 2 (5.3) | 2 (14.3) | 0 (0) | 2 (25) | 0 (0) | 0 (0) |
| IVIg | 5 (6.3) | 2 (5.3) | 2 (14.3) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) |
Abbreviation: AIDS = acquired immunodeficiency syndrome; HIV = human immunodeficiency virus; ICI = immune checkpoint inhibitor; IQ25–75 = interquartile range.
Factors Associated with 1‐Year Survival, and Indirect Indicators of Biological Activity of ICIs
One‐year survival following ICI initiation was 51.9% and was similar across groups (Table 3), with the exception of the 2 kidney transplant recipients, who died from PML 19 and 20 days after ICI initiation. The 1‐year probability of survival following ICI initiation is shown in Figure 1A for the whole cohort and in Figure 1B based on underlying conditions. Overall, 38 of 79 patients died (48.1%). Cause of death was attributed to PML for 26 of 38 patients (68.4%), ranging from 1 of 3 deaths in the group with chronic inflammatory diseases (33.3%) to 15 of 19 deaths in the group with hematological disorders (79.9%), not considering the 2 smaller groups of patients with solid neoplasm and kidney transplantation. Fatal outcome was attributed to PML‐IRIS in 7 of 38 patients (18.4%), ranging from 3 of 19 (15.8%) deaths in the group with hematological disorders to 2 of 3 deaths in the group with chronic inflammatory diseases (66.7%). Finally, for 3 of 38 (7.9%) and 2 of 38 (5.3%), death was attributed respectively to the progression of both PML and the underlying disease, or of the underlying disease only. Among patients who survived PML, the median mRS was 3 (IQ25–75 = 2–4) at ICI initiation and 3 (IQ25–75 = 2–3.5) at the last available follow‐up (at median 365 days after ICI initiation [IQ25–75 = 251–646]). We considered development of contrast enhancement by MRI, occurrence of PML‐IRIS, and a decrease of >1 log copies/ml of CSF JCV DNA copy number at least once during follow‐up as indirect indicators of biological activity of ICI. Forty‐eight of 79 patients (60.8%) presented at least one of these parameters, suggestive of the reinvigoration of immune responses, ranging from 0 of 2 kidney transplant recipients to 6 of 8 patients with chronic inflammatory disease (75%).
TABLE 3.
Survival, causes of death at 12 months, and indirect indicators of biological activity of ICIs
| All, n = 79 | Hematological Malignancies, n = 38 | Primary Immunodeficiencies, n = 14 | HIV/AIDS, n = 12 | Chronic Inflammatory Diseases, n = 8 | Solid Neoplasm, n = 5 | Transplant Recipients, n = 2 | |
|---|---|---|---|---|---|---|---|
| Survivors at 12 months after ICI initiation, n (%) | 41 (51.9) | 19 (50) | 8 (57.1) | 6 (50) | 5 (62.5) | 3 (60) | 0 (0) |
| Death from any cause, n (%) | 38 (48.1) | 19 (50) | 6 (42.9) | 6 (50) | 3 (37.5) | 2 (40) | 2 (100) |
| Death from PML, n (%) | 26 (32.9) | 15 (38.5) | 4 (28.6) | 2 (16.7) | 1 (12.5) | 2 (40) | 2 (100) |
| Death from PML‐IRIS, n (%) | 7 (8.9) | 3 (7.7) | — | 2 (16.7) | 2 (25) | — | — |
| Death from PML and underlying condition, n (%) a | 3 (3.8) | — | 1 (7.1) | 2 (16.7) | — | — | — |
| Death from underlying condition, n (%) b | 2 (2.5) | 1 (2.6) | 1 (7.1) | — | — | — | — |
| Time between ICI initiation and death, days, median (IQ25–75) | 56 (31.3–74.8) | 56 (38–84) | 162 (84–231.8) | 48 (28–58.3) | 21 (20.5–40.5) | 23.5 (17.8–29.3) | 19.5 (19.3–19.8) |
| MRI contrast‐enhancing lesion at least once during follow‐up, n (%) |
32 (45.1) [71/79] |
15 (42.9) [35/38] |
7 (50) |
6 (60) [10/12] |
4 (66.7) [6/8] |
0 [4/5] |
0 |
| PML‐IRIS during follow‐up, n (%) | 15 (19) | 6 (15.8) | 1 (7.1) | 5 (41.7) | 3 (37.5) | 0 | 0 |
| >1 log copies/ml CSF JCV DNA decrease at least once during follow‐up, n (%) |
30 (58.8) [51/79] |
12 (52.2) [23/38] |
7 (63.6) [11/14] |
6 (60) [10/12] |
3 (75) [4/8] |
2 (66.7) [3/5] |
— |
| Any of the 3 surrogate markers of an effect of the therapy, n (%) | 48 (60.8) | 22 (57.9) | 10 (71.4) | 8 (66.7) | 6 (75) | 2 (40) | 0 |
In cases of missing data, the actual number of patients is indicated in brackets after each result.
One patient with a primary immunodeficiency and 2 people living with HIV died from sepsis while PML was worsening.
One patient with diffuse large B‐cell lymphoma died from relapse, and 1 patient with a primary immunodeficiency died from toxoplasma encephalitis while PML was stable.
Abbreviation: AIDS = acquired immunodeficiency syndrome; CSF = cerebrospinal fluid; HIV = human immunodeficiency virus; ICI = immune checkpoint inhibitor; IRIS = immune reconstitution inflammatory syndrome; JCV = JC polyomavirus; MRI = magnetic resonance imaging; PML = progressive multifocal leukoencephalopathy.
FIGURE 1.
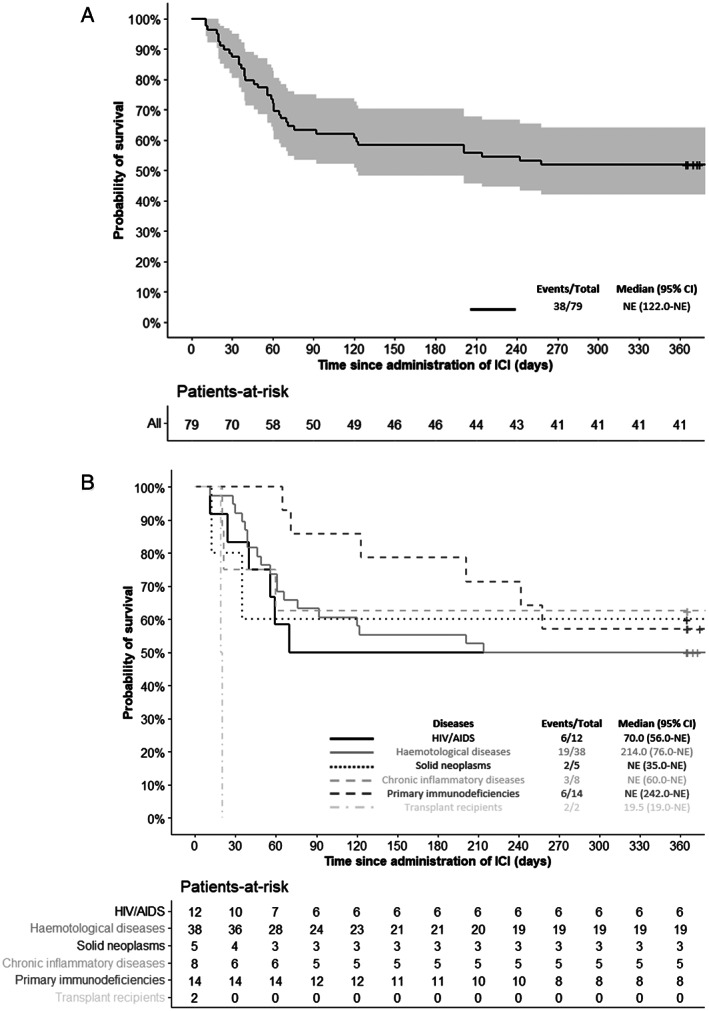
One‐year probability of survival following immune checkpoint inhibitor (ICI) initiation. (A) One‐year probability of survival following ICI initiation in the whole cohort (light gray area indicates 95% confidence interval [CI]). (B) One‐year probability of survival following ICI according to underlying diseases. AIDS = acquired immunodeficiency syndrome; HIV = human immunodeficiency virus; NE = non existent.
Associations of patients' characteristics at ICI initiation with 1‐year survival are presented in Table 4. In multivariate analysis, none of the variables considered was associated with survival, including demographic features (sex, age), PML‐predisposing condition, time from symptom onset to PML diagnosis and ICI initiation, treatment history (history of immunosuppressive treatment, type of ICI used), measures of disease severity (extent of MRI lesion burden or clinical disability accrued), and immune status (blood lymphocyte and T‐cell counts). Detection of contrast‐enhancing lesions on baseline brain MRI was associated with a trend toward better survival (odds ratio [OR] = 3.13, 95% confidence interval [CI] = 0.93–12.00, p = 0.07).
TABLE 4.
Univariate and Multivariate Analyses of Variables at ICI Initiation Associated with 1‐Year Survival
| Variable | Univariate Analysis, n = 64 a | Multivariate Analysis Adjusted on Sex and Age | ||||
|---|---|---|---|---|---|---|
| Survivors, n = 34 | Nonsurvivors, n = 30 | OR (95% CI) | p | OR (95% CI) | p | |
| Sex, n (%) | ||||||
| Female | 8 (42.1) | 11 (57.9) | 1 | 0.25 | ||
| Male | 26 (57.8) | 19 (42.2) | 1.88 (0.64–5.74) | |||
| Age at PML diagnosis, yr, median (IQ25–75) | 60.5 (49.3–70) | 59 (50.3–70.8) | 0.99 (0.96–1.03) | 0.75 | ||
| PML‐related condition, n (%) | ||||||
| Hematological malignancies | 13 (50) | 13 (50) | 0.48 | |||
| Primary immunodeficiencies | 8 (57.1) | 6 (42.9) | ||||
| HIV/AIDS | 6 (50) | 6 (50) | ||||
| Chronic inflammatory diseases | 4 (66.7) | 2 (33.3) | ||||
| Solid neoplasm | 3 (75) | 1 (25) | ||||
| Transplant recipient | 0 (0) | 2 (100) | ||||
| Time from symptom onset to PML diagnosis, days, median (IQ25–75) | 56 (22–77) | 31.5 (18.3–62.5) | 1.01 (1.00–1.02) | 0.21 | ||
| Time from PML diagnosis to ICI initiation, days, median (IQ25–75) | 26 (16.5–42.5) | 23 (13.3–42.5) | 1.00 (1.00–1.02) | 0.35 | ||
| Immunosuppressive treatment before PML diagnosis, n (%) | ||||||
| Yes | 16 (45.7) | 19 (54.3) | 0.51 (0.18–1.39) | 0.19 | 0.40 (0.11–1.31) | 0.14 |
| No | 18 (62.1) | 11 (37.9) | 1 | |||
| mRS, median (IQ25–75) | 3 (2–4) | 4 (3–4) | 0.67 (0.40–1.06) | 0.10 | 0.74 (0.43–1.25) | 0.26 |
| Brain regions involved by PML, n, median (IQ25–75) | 3 (2–3.8) | 3 (2–4) | 1.00 (0.76–1.31) | 0.98 | ||
| Contrast‐enhancing lesion on brain MRI, n (%) | ||||||
| Yes | 12 (70.6) | 5 (29.4) | 2.73 (0.86–9.71) | 0.10 | 3.13 (0.93–12.1) | 0.07 |
| No | 22 (46.8) | 25 (53.2) | 1 | |||
| Lymphocyte count per μl, median (IQ25–75) | 1,015 (573–1,456) | 795 (490–1,100) | 1.00 | 0.25 | ||
| CD4+ T‐cell count per μl, median (IQ25–75) | 195 (120–353) | 161 (95–292) | 1.00 | 0.89 | ||
| CD8+ T‐cell count per μl, median (IQ25–75) | 235 (117–731) | 295 (71–461) | 1.00 | 0.29 | ||
| Type of ICI, n (%) | ||||||
| Pembrolizumab | 24 (57.1) | 18 (42.9) | 0.67 | |||
| Nivolumab | 9 (45) | 11 (55) | ||||
| Atezolizumab | 1 (50) | 1 (50) | ||||
Analyses were performed in a subset of 64 patients with no missing data at baseline, 34 survivors and 30 nonsurvivors.
Abbreviation: AIDS = acquired immunodeficiency syndrome; CI = confidence interval; HIV = human immunodeficiency virus; ICI = immune checkpoint inhibitor; IQ25–75 = interquartile range; MRI = magnetic resonance imaging; mRS = modified Rankin Scale; OR = odds ratio; PML = progressive multifocal leukoencephalopathy.
For several variables collected at baseline and during the 12‐month follow‐up, missing data precluded the possibility of performing analysis other than exploratory univariate analysis (Table S2). Survival was not associated with the recovery of blood lymphocyte and CD4+ or CD8+ T‐cell counts during the first month after ICI initiation, nor with pretreatment percentage of PD‐1‐expressing peripheral blood CD4+ or CD8+ T cells, nor with a decrease in the proportion of circulating PD‐1‐expressing T cells to <5%. Although numbers may indicate a trend, detectable JCV‐specific immune responses at baseline or during the follow‐up were not significantly associated with survival. However, presence of contrast enhancement on at least one brain MRI during follow‐up was associated with higher odds of survival (3.16, 95% CI = 1.20–8.72, p = 0.02). Decrease of >1 log copies/ml in CSF JCV DNA load from treatment to first follow‐up after ICI initiation was not associated with survival (OR = 2.6, 95% CI = 0.75–9.85, p = 0.15), although CSF JCV DNA load decreased significantly by 1 month post‐treatment in survivors compared to nonsurvivors (p < 0.0001; Fig 2).
FIGURE 2.
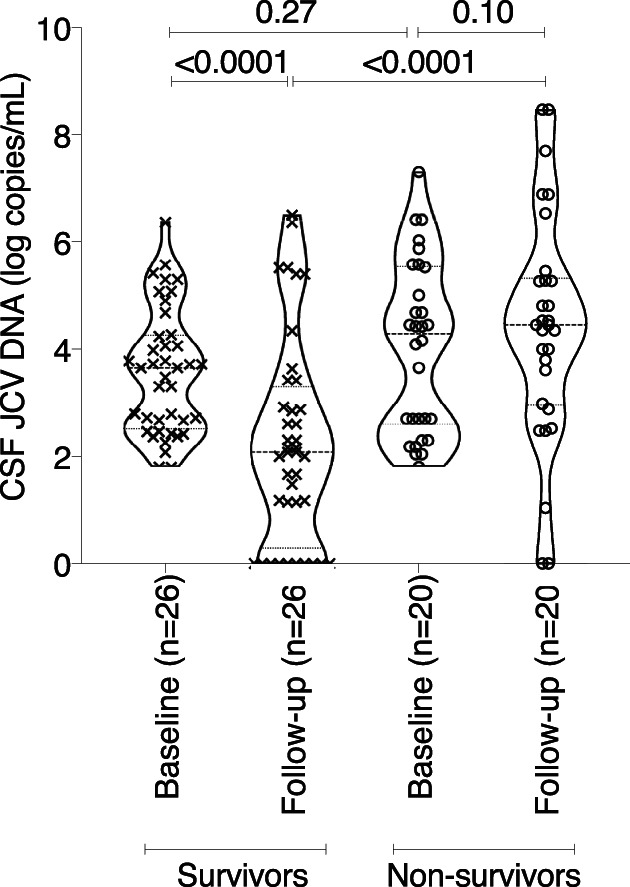
Cerebrospinal fluid (CSF) JC polyomavirus (JCV) DNA at baseline and at first follow‐up after immune checkpoint inhibitor (ICI) initiation between survivors and nonsurvivors. Paired CSF JCV DNA at ICI initiation and at first follow‐up performed after median delay of 27 days (interquartile range = 20–33) after ICI initiation is shown among 46 patients, 26 survivors, and 20 nonsurvivors (Wilcoxon Mann–Whitney tests were used to compare survivors and nonsurvivors at the same timepoints, whereas Wilcoxon signed‐rank tests for paired data were used for baseline and follow‐up comparison).
Safety
Thirty‐two adverse events were identified in 24 patients (30.4%; Table 5). The majority were off‐target immune‐mediated dermatological (9/24, 37.5%) and gastrointestinal (5/24, 20.8%) manifestations, and infusion‐related adverse events (7/24, 29.2%). Patients who developed adverse events had a trend for having received more ICI infusions (median = 3, IQ25–75 = 2–5) compared to those who did not (median = 2, IQ25–75 = 2–4, p = 0.055). Adverse events led to discontinuation of treatment in 7 of 24 patients (29.1%).
TABLE 5.
Adverse Events of Immune Checkpoint Inhibitor Treatment
| Adverse Event | All, n = 79 | Hematological Malignancies, n = 38 | Primary Immunodeficiencies, n = 14 | HIV/AIDS, n = 12 | Chronic Inflammatory Diseases, n = 8 | Solid Neoplasm, n = 5 | Transplant Recipients, n = 2 |
|---|---|---|---|---|---|---|---|
| Patients with side effects, n (%) [32 adverse events in 24 patients] | 24 (30.4) | 10 (26.3) | 10 (71.4) | 2 (16.7) | 1 (12.5) | 1 (20) | 0 (0) |
| Dermatological manifestations [rash, psoriasis, vitiligo], n (%) | 9/24 (37.5) | 4 | 5 | — | — | — | — |
| Infusion‐related side effects [fever, asthenia, anaphylaxis], n (%) | 7/24 (29.2) | 1 | 4 | 2 | — | — | — |
| Gastrointestinal manifestation [vomiting, diarrhea, colitis, mucositis], n (%) | 5/24 (20.8) | 2 | 3 | — | — | — | — |
| Cytopenia, n (%) | 2/24 (8.3) | 1 | 1 | — | — | — | — |
| Hepatitis, n (%) | 2/24 (8.3) | 1 | 1 | — | — | — | — |
| Myositis, n (%) | 1/24 (4.2) | 1 | — | — | — | — | — |
| Pneumonitis, n (%) | 2/24 (8.3) | 1 | — | — | 1 | — | — |
| Dysthyroidism, n (%) | 1/24 (4.2) | 1 | — | — | — | — | — |
| Autoimmune cardiac toxicity/heart failure, n (%) | 2/24 (8.3) | 2 | — | — | — | — | — |
| Arthritis, n (%) | 1/24 (4.2) | — | — | — | — | 1 | — |
Abbreviation: AIDS = acquired immunodeficiency syndrome; HIV = human immunodeficiency virus.
PML‐IRIS occurred in 15 of 79 patients (19%) with a median delay after ICI initiation of 25 days (IQ25–75 = 13.3–34.8, 1 missing data); by definition, PML‐IRIS was associated with clinical worsening (median mRS increase of 1 [IQ25–75 = 1–1]). Among cases of PML‐IRIS, 8 were undergoing immunosuppressive treatment at PML diagnosis (53.3%), which was subsequently stopped in 6 of 8 patients. The 5 HIV/AIDS patients who developed PML‐IRIS had initiated or optimized ART regimen a median of 86 days (IQ25–75 = 31–114, 1 missing data) prior to first ICI infusion. PML‐IRIS was treated with corticosteroids in 9 of 15 patients (60%), and ICI was discontinued in 8 (53.3%). Of note, PML‐IRIS occurred irrespective of the presence of contrast‐enhancing brain MRI lesions before ICI initiation (7/21 [33.3%] with contrast‐enhancing lesions vs 8/58 [13.8%] without, chi‐squared test, p = 0.1).
Discussion
In this large multicenter retrospective “real‐world” survey of patients with PML related to various underlying conditions who were treated with ICIs as add‐on to standard of care, the overall survival at 12 months following ICI initiation was 51.9%.
With no antiviral drug available against JCV, standard of care of PML aims to restore antiviral immune responses by directly addressing the underlying immune suppression. 1 When this is not readily achieved, such as in patients with hematological malignancy, 13 transplant recipients, 14 and patients with primary immunodeficiency, 15 1‐year case fatality rates remain high, ranging from 85 to 90%. On the other hand, mortality is substantially lower among patients with HIV/AIDS since the combined ART era (ie, 25–50%), 16 , 17 and among patients with multiple sclerosis following discontinuation of the immunomodulatory drug natalizumab (ie, 24%). 18 The raw effect of ICI add‐on to standard of care on survival is difficult to ascertain in a noncontrolled study. Similarly to what we observed in a recently published survey of patients with PML treated with recombinant human IL‐7, 2 1‐year survival in HIV/AIDS patients (50%) did not differ from that reported for standard of care, although, as for the other patients in this study, a selection bias accounting for the decision to pursue ICIs as add‐on to ART cannot be excluded. In this study, survival of patients with hematological malignancy (50%) and primary immunodeficiency (57.1%) was greater than historically reported, suggesting a potential benefit of ICI add‐on to standard of care. However, it is also possible that survival of "historical controls" underestimates current survival rates, as suggested by a recent nationwide study in France that found 1‐year survival following PML diagnosis of patients with hematological malignancies and primary immune deficiencies was respectively of 39.2 and 55.6%. 19 Of note, the significantly longer delay between ICI initiation and death in patients with primary immune deficiencies as compared to the other categories is an interesting observation; although the molecular heterogeneity of this subcohort precludes generalizable conclusions, further investigation into this finding may provide greater insight into mechanisms contributing to immune exhaustion and susceptibility to rescue using ICIs. The low numbers of patients in this study with underlying chronic inflammatory disease, solid neoplasm, and kidney transplantation limit interpretation in these groups.
Survival in PML is affected by progression of the underlying disease, the extent of neurological injury sustained, and, when immune reconstitution is achieved, the potential deleterious effects of uncontrolled inflammation. Furthermore, compared to direct antiviral strategies, effectiveness of interventions that leverage intrinsic immune responses may differ greatly across underlying immune deficits. All these factors complicate the interpretation of impact of ICIs on PML disease. In an attempt to tease out these different elements, in addition to overall survival, we have considered additional intermediate outcomes that may indirectly support reinvigoration of antiviral immune responses by ICIs, including detection of inflammation by MRI and CSF JCV viral load. Independently of underlying disease, the detection of CD4+ and cytotoxic CD8+ T‐cell reactivity against JCV in blood, CSF, or brain correlates with subsequent control of PML and survival. 16 , 17 , 20 , 21 , 22 , 23 , 24 In the present work, although limited by the low number of patients and the lack of standardization of methods used to probe virus‐specific T‐cell reactivity, the trend observed of better survival in patients having detectable anti‐JCV immune responses is of interest. Instead, we considered indirect measures of recovery of JCV‐specific T‐cell immune responses as possibly providing hints of the desired biological effect of ICI therapy. PML patients with detectable cytotoxic CD8+ T‐cell reactivity against JCV have been shown to have an increased likelihood of having contrast enhancement of brain MRI PML lesions, 17 and this imaging biomarker is predictive of prolonged survival in HIV/AIDS patients with PML. 25 In this study, contrast enhancement of PML lesions was significantly associated in univariate analysis with higher survival when detected at least once during the follow‐up. Massively abundant in PML‐IRIS lesions, cytotoxic CD8+ T cells are also considered drivers of PML‐IRIS. 26 , 27 In our survey, PML‐IRIS occurred in a substantial proportion of patients (19%), reaching 37.5% and 41.7% in patients with chronic inflammatory diseases and HIV/AIDS‐related PML, which is higher than previously reported in HIV/AIDS PML patients, 28 , 29 suggesting that ICIs may have intensified recovery of immune responses. Although CSF JCV DNA load at the time of ICI initiation was similar across patients, values were decreased at 1‐month follow‐up in survivors only. Clearance (or decrease) of JCV from CSF, mediated by recovered immune responses, has been shown in HIV/AIDS PML patients to be a strong predictor for survival. 30 , 31 , 32 Finally, death was attributed to PML by the treating physician in 32.9% of patients, which is in line with the findings of 2 recent reviews compiling small series and single cases of PML treated by ICIs. 33 , 34 Thus, although overall survival as a primary endpoint is straightforward and relevant, it is important to recognize that the 48.1% all‐cause mortality may miss some of the complexities in this disease.
The evidence for use of ICIs in PML requires further development. Immune exhaustion appears as a shared mechanism of chronic infections and tumors limiting T‐cell responses. 10 , 35 ICI therapy in animal models of lymphocytic choriomeningitis virus, hepatitis B virus and simian immunodeficiency virus infections, and in patients living with HIV, resulted in expansion of virus‐specific CD4+ and CD8+ T cells with improved function, 9 , 36 , 37 suggesting a therapeutic role for chronic viral infections. 38 , 39 Several findings suggested a role of immune exhaustion in chronic JCV infection leading to PML, and a potential place for ICI therapy. 4 , 40 , 41 , 42 , 43 Nevertheless, these data should not be overinterpreted. First, functional effector T cells also express inhibitory receptors, such that expression of PD‐1 alone is not sufficient to identify immune exhaustion. The proof of T‐cell dysfunctionality in PML, based on the operational definition of immune exhaustion, still has to be made. 8 Second, immune exhaustion encompasses multiple differentiation stages, including phenotypically and functionally heterogeneous subsets of exhausted T cells. Among them, memorylike exhausted T cells defined by the expression of the transcription factor T‐cell factor 1 were shown to be critical for reinvigoration of antigen‐specific responses in response to ICIs during chronic viral infection. 44 , 45 This population may represent a prime target for therapeutic interventions to improve the immune response in chronic infections, and further studies aiming to determine the role of specific subpopulations in mediating response to ICIs, and to identify predictors of response are needed. 46
A salient point was the incidence of PML‐IRIS. Although the largest case series to date did not report PML‐IRIS among the 8 treated patients, except possibly for one, 4 and although PML‐IRIS was sparsely noted by case reports, 15 patients (19%) in our survey presented with clinical worsening and new or worsening contrast‐enhancing lesions and/or edema on brain MRI. Seven were considered to have died from PML‐IRIS, highlighting the importance of weighing the risk of PML‐IRIS before undertaking treatment with ICIs, particularly in patients with HIV/AIDS and chronic inflammatory diseases, two subgroups in which immune recovery may be more readily achieved through ART initiation or the discontinuation of immunosuppressive medications. Improved detection and diagnosis of PML‐IRIS 47 , 48 as well as controlled studies to determine optimal management of PML‐IRIS are needed. 49 , 50 Other than PML‐IRIS, adverse effects occurred in 30.4% of patients, leading to the discontinuation of ICI in 29.1% of these patients. Interestingly, patients with primary immunodeficiency, who received the highest number of ICI infusions, were the most affected, with 71.4% of patients experiencing at least one adverse event.
This work is limited by its observational, retrospective, and noncomparative design. The population was heterogenous, with different underlying conditions and subgroups of very small size, which limits any extrapolation. In addition, 38 cases of our cohort were already published cases, which may represent a publication bias of positive cases that we tried to counterbalance by extending the survey to nonpublished cases. Furthermore, case selection may have been subject to referral bias to expert centers of particularly severe patients requiring additional intervention as add‐on to standard of care, or conversely to less severe patients who survived long enough to receive this emerging treatment. The study was multicentric, and therefore analyses were not standardized among centers, including CSF JCV DNA detection and quantification, flow‐activated cell sorting analysis of PD‐1 expression, and MRI evaluations. Along the same line, statistical analysis was limited by the small sample size and missing data. Given the retrospective design of the study, we cannot exclude underreporting of ICI‐related adverse events, and were unable to grade events. On the other hand, this unique real‐world study includes a large number of patients with definite PML who received ICIs, and with clinical and laboratory data collected in a standardized CRF, to guarantee, as much as possible, the validity of the information.
In conclusion, this study provides important insights into the effects of immunotherapeutic intervention with ICI for PML. Although small numbers and lack of direct comparisons do not permit definitive conclusions, it is possible that ICIs may have supported reinvigoration of antiviral immune responses, with a survival benefit in some patients. Prospective controlled clinical trials will be needed to provide firm indications for this therapeutic strategy in clinical practice. Until then, the authors would emphasize that management of PML should be strictly personalized, weighing the underlying predisposing condition, the likelihood of adequate response to standard of care, and the specific risk of PML‐IRIS and off‐target adverse events.
Author Contributions
G.M.‐B. and I.C. contributed to the conception and design of the study. X.B., B.B., D.R.‐W., C.P., S.R., L.N., A.D.B., I.J., K.‐W.S., J.G., Y.G., O.G., S.G., R.W., N.L., T.P., M.B., D.C., I.C., and G.M.‐B. contributed to the acquisition and analysis of data. X.B., B.B., A.S., N.L., I.C., and G.M.‐B. contributed to drafting the text and preparing the figures. The members of the Immunotherapy for PML Study Group contributed to the acquisition of data, and can be found in Table S1.
Potential Conflicts of Interest
Nothing to report.
Supporting information
APPENDIX S1: Supporting Information
TABLE S1. Immunotherapy for PML Study Group Members and Affiliations
TABLE S2. Univariate Analyses of Other Variables at ICI Initiation and During Follow‐up for Their Association with 1‐Year Survival
Acknowledgments
We thank patients and their relatives, and caring nurses and physicians. This study was not funded. I.C. is supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program.
Contributor Information
Guillaume Martin‐Blondel, Email: martin-blondel.g@chu-toulouse.fr.
for the Immunotherapy for PML Study Group:
Bodo Grimbacher, Clemens Warnke, Rebecca Wicklein, Martijn Wijburg, Matthijs Brouwer, Nicolas Lambert, Xavier Engalenc, Marion Gaudin, Clemens Küpper, Achille Aouba, Victoria Manda, Xavier Brousse, Maïlys Ducours, Pierre Duffau, Jean‐Christophe Ouallet, Hela Mrabet, Florence Gourdon, Marion Le Maréchal, Raphael Bernard‐Valnet, Pierre Delobel, Rebecca Lajaunie, Emmanuel Treiner, Sebastien Lhomme, Fabrice Bonneville, Carole Ribaute, Jonathan Ciron, Damien Biotti, Nassim Kamar, Nicolas Weiss, Valérie Pourcher, Juliette Rakotoarison, Yann Leveneur, Laurence De Menibus, Niklas Grassl, Francois Lifermann, Fleur Cohen‐Aubart, Douglas Ney, Ronak Kapadia, Yael Dinur‐Schejter, Tzlil Shifman, Oded Shamriz, Joseph Berger, Olivier Lambotte, Thomas Perpoint, Asaff Harel, and Benjamin Wyplosz
Data Availability Statement
Data requests should be sent to G.M.‐B. Data access must be approved by the French data protection authority, la Commission Nationale de l'Informatique et des Libertés. For further information, please see https://www.cnil.fr
References
- 1. Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus‐related disease. Nat Rev Neurol 2021;17:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lajaunie R, Mainardi I, Gasnault J, et al. Outcome of progressive multifocal leukoencephalopathy treated by Interleukin‐7. Ann Neurol 2022;91:496–505. [DOI] [PubMed] [Google Scholar]
- 3. Cortese I, Beck ES, Al‐Louzi O, et al. BK virus‐specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: an open‐label, single‐cohort pilot study. Lancet Neurol 2021;20:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cortese I, Muranski P, Enose‐Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med 2019;380:1597–1605. [DOI] [PubMed] [Google Scholar]
- 5. Walter O, Treiner E, Bonneville F, et al. Treatment of progressive multifocal leukoencephalopathy with nivolumab. N Engl J Med 2019;380:1674–1676. [DOI] [PubMed] [Google Scholar]
- 6. Boussiotis VA. Molecular and biochemical aspects of the PD‐1 checkpoint pathway. N Engl J Med 2016;375:1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schonrich G, Raftery MJ. The PD‐1/PD‐L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol 2019;9:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blank CU, Haining WN, Held W, et al. Defining 'T cell exhaustion'. Nat Rev Immunol 2019;19:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gay CL, Bosch RJ, Ritz J, et al. Clinical trial of the anti‐PD‐L1 antibody BMS‐936559 in HIV‐1 infected participants on suppressive antiretroviral therapy. J Infect Dis 2017;215:1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 2018;18:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin‐Blondel G, Delobel P, Blancher A, et al. Pathogenesis of the immune reconstitution inflammatory syndrome affecting the central nervous system in patients infected with HIV. Brain 2011;134:928–946. [DOI] [PubMed] [Google Scholar]
- 12. Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adrianzen Herrera D, Ayyappan S, Jasra S, et al. Characteristics and outcomes of progressive multifocal leukoencephalopathy in hematologic malignancies and stem cell transplant—a case series. Leuk Lymphoma 2019;60:395–401. [DOI] [PubMed] [Google Scholar]
- 14. Mateen FJ, Muralidharan R, Carone M, et al. Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol 2011;70:305–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zerbe CS, Marciano BE, Katial RK, et al. Progressive multifocal leukoencephalopathy in primary immune deficiencies: Stat1 gain of function and review of the literature. Clin Infect Dis 2016;62:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gasnault J, Costagliola D, Hendel‐Chavez H, et al. Improved survival of HIV‐1‐infected patients with progressive multifocal leukoencephalopathy receiving early 5‐drug combination antiretroviral therapy. PLoS One 2011;6:e20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology 2009;73:1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Medical information. TYSABRI (natalizumab): PML incidence in patients receiving natalizumab. Cambridge, MA: Biogen , 2022.
- 19. Joly M, Conte C, Cazanave C, et al. Progressive multifocal leukoencephalopathy: epidemiology and spectrum of predisposing conditions. Brain 2022;awac237. [DOI] [PubMed] [Google Scholar]
- 20. Aly L, Yousef S, Schippling S, et al. Central role of JC virus‐specific CD4+ lymphocytes in progressive multi‐focal leucoencephalopathy‐immune reconstitution inflammatory syndrome. Brain 2011;134:2687–2702. [DOI] [PubMed] [Google Scholar]
- 21. Du Pasquier RA, Autissier P, Zheng Y, et al. Presence of JC virus‐specific CTL in the cerebrospinal fluid of PML patients: rationale for immune‐based therapeutic strategies. AIDS 2005;19:2069–2076. [DOI] [PubMed] [Google Scholar]
- 22. Du Pasquier RA, Kuroda MJ, Zheng Y, et al. A prospective study demonstrates an association between JC virus‐specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain 2004;127:1970–1978. [DOI] [PubMed] [Google Scholar]
- 23. Gasnault J, Kahraman M, de Goer de Herve MG, et al. Critical role of JC virus‐specific CD4 T‐cell responses in preventing progressive multifocal leukoencephalopathy. AIDS 2003;17:1443–1449. [DOI] [PubMed] [Google Scholar]
- 24. Lima MA, Marzocchetti A, Autissier P, et al. Frequency and phenotype of JC virus‐specific CD8+ T lymphocytes in the peripheral blood of patients with progressive multifocal leukoencephalopathy. J Virol 2007;81:3361–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berger JR, Levy RM, Flomenhoft D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome‐associated progressive multifocal leukoencephalopathy. Ann Neurol 1998;44:341–349. [DOI] [PubMed] [Google Scholar]
- 26. Martin‐Blondel G, Bauer J, Cuvinciuc V, et al. In situ evidence of JC virus control by CD8+ T cells in PML‐IRIS during HIV infection. Neurology 2013;81:964–970. [DOI] [PubMed] [Google Scholar]
- 27. Metz I, Radue EW, Oterino A, et al. Pathology of immune reconstitution inflammatory syndrome in multiple sclerosis with natalizumab‐associated progressive multifocal leukoencephalopathy. Acta Neuropathol 2012;123:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muller M, Wandel S, Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta‐analysis. Lancet Infect Dis 2010;10:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sainz‐de‐la‐Maza S, Casado JL, Perez‐Elias MJ, et al. Incidence and prognosis of immune reconstitution inflammatory syndrome in HIV‐associated progressive multifocal leucoencephalopathy. Eur J Neurol 2016;23:919–925. [DOI] [PubMed] [Google Scholar]
- 30. Bossolasco S, Calori G, Moretti F, et al. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV‐associated progressive multifocal leukoencephalopathy. Clin Infect Dis 2005;40:738–744. [DOI] [PubMed] [Google Scholar]
- 31. De Luca A, Giancola ML, Ammassari A, et al. The effect of potent antiretroviral therapy and JC virus load in cerebrospinal fluid on clinical outcome of patients with AIDS‐associated progressive multifocal leukoencephalopathy. J Infect Dis 2000;182:1077–1083. [DOI] [PubMed] [Google Scholar]
- 32. Giudici B, Vaz B, Bossolasco S, et al. Highly active antiretroviral therapy and progressive multifocal leukoencephalopathy: effects on cerebrospinal fluid markers of JC virus replication and immune response. Clin Infect Dis 2000;30:95–99. [DOI] [PubMed] [Google Scholar]
- 33. Bernard‐Valnet R, Koralnik IJ, Du Pasquier R. Advances in treatment of progressive multifocal leukoencephalopathy. Ann Neurol 2021;90:865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohn N, Grote‐Levi L, Hopfner F, et al. Innovative therapeutic concepts of progressive multifocal leukoencephalopathy. J Neurol 2022;269:2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med 2014;371:380–383. [DOI] [PubMed] [Google Scholar]
- 36. Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682–687. [DOI] [PubMed] [Google Scholar]
- 37. Velu V, Titanji K, Zhu B, et al. Enhancing SIV‐specific immunity in vivo by PD‐1 blockade. Nature 2009;458:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chomont N, El‐Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009;15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu J, Zhang E, Ma Z, et al. Enhancing virus‐specific immunity in vivo by combining therapeutic vaccination and PD‐L1 blockade in chronic hepadnaviral infection. PLoS Pathog 2014;10:e1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Audemard‐Verger A, Gasnault J, Faisant M, et al. Sustained response and rationale of programmed cell death‐1‐targeting for progressive multifocal leukoencephalopathy. Open Forum Infect Dis 2019;6:ofz374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garcia J, Hendel‐Chavez H, De‐Goer MG, et al. Progressive multifocal leukoencephalopathy on dimethyl fumarate with preserved lymphocyte count but deep T‐cells exhaustion. Mult Scler 2021;27:640–644. [DOI] [PubMed] [Google Scholar]
- 42. Tan CS, Bord E, Broge TA Jr, et al. Increased program cell death‐1 expression on T lymphocytes of patients with progressive multifocal leukoencephalopathy. J Acquir Immune Defic Syndr 2012;60:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tan CS, Broge TA Jr, Seung E, et al. Detection of JC virus‐specific immune responses in a novel humanized mouse model. PLoS One 2013;8:e64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD‐1 therapy. Nature 2016;537:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Utzschneider DT, Charmoy M, Chennupati V, et al. T cell factor 1‐expressing memory‐like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity 2016;45:415–427. [DOI] [PubMed] [Google Scholar]
- 46. Beck ES, Cortese I. Checkpoint inhibitors for the treatment of JC virus‐related progressive multifocal leukoencephalopathy. Curr Opin Virol 2020;40:19–27. [DOI] [PubMed] [Google Scholar]
- 47. Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T‐cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol 2011;85:7256–7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gheuens S, Ngo L, Wang X, et al. Metabolic profile of PML lesions in patients with and without IRIS: an observational study. Neurology 2012;79:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bernard‐Valnet R, Moisset X, Maubeuge N, et al. CCR5 blockade in inflammatory PML and PML‐IRIS associated with chronic inflammatory diseases' treatments. Neurol Neuroimmunol Neuroinflamm 2022;9:e1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tan K, Roda R, Ostrow L, et al. PML‐IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 2009;72:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1: Supporting Information
TABLE S1. Immunotherapy for PML Study Group Members and Affiliations
TABLE S2. Univariate Analyses of Other Variables at ICI Initiation and During Follow‐up for Their Association with 1‐Year Survival
Data Availability Statement
Data requests should be sent to G.M.‐B. Data access must be approved by the French data protection authority, la Commission Nationale de l'Informatique et des Libertés. For further information, please see https://www.cnil.fr


