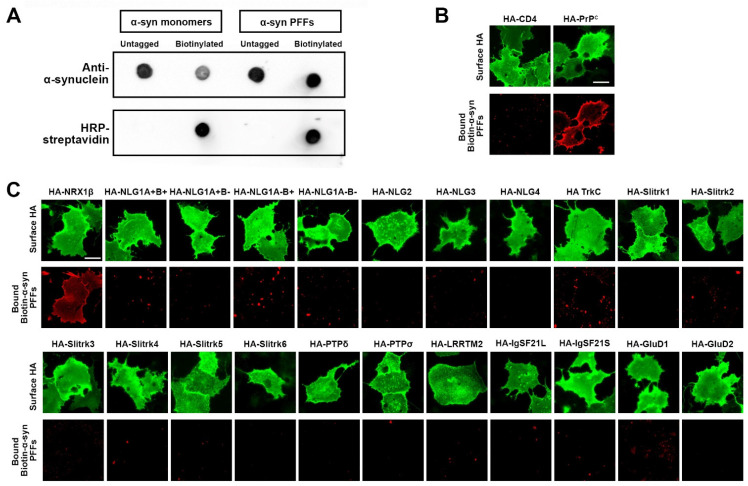
Figure 2.
A candidate screen based on cell surface protein binding assays using biotin-conjugated α-syn PFFs isolates neurexin1β (NRX1β) as an α-syn PFF-interacting protein. (A) Validation of biotin conjugation to α-syn monomers and PFFs by dot blot assays. Untagged and biotin-conjugated α-syn monomers and PFFs were spotted onto a nitrocellulose membrane and immunolabeled with anti-α-synuclein antibody to confirm the presence of the indicated proteins. After stripping the anti-α-synuclein antibody, the membrane was labelled with HRP-conjugated streptavidin to detect biotin-conjugated proteins. Only biotin-α-syn monomers and PFFs, but not untagged ones, display HRP-streptavidin-based signals. (B) A cell surface protein binding assay shows that the biotin-α-syn PFFs bind to COS-7 cells expressing the N-terminal extracellular HA-tagged cellular prion protein (HA-PrPc), which is a known α-syn PFF-interacting protein, but not those expressing HA-CD4 as a negative control. The surface HA was immunolabelled to verify the expression of these constructs on the COS-7 cell surface. Scale bar: 30 μm. (C) Representative images showing cell surface protein binding assays testing for interaction between α-syn PFFs (1 µM, monomer equivalent) and known synaptic organizers. COS-7 cells expressing the indicated construct were exposed to α-syn PFFs. α-syn PFFs bind to COS-7 cells expressing HA-tagged neurexin 1β (HA-NRX1β), but not to those expressing any of the other organizers including HA-neuroligin1 (HA-NLG1). Surface HA (green) was immunostained to verify the expression of the indicated N-terminal extracellular HA-tagged constructs on the COS-7 cell surface. Scale bar: 30 μm.