Figure 3.
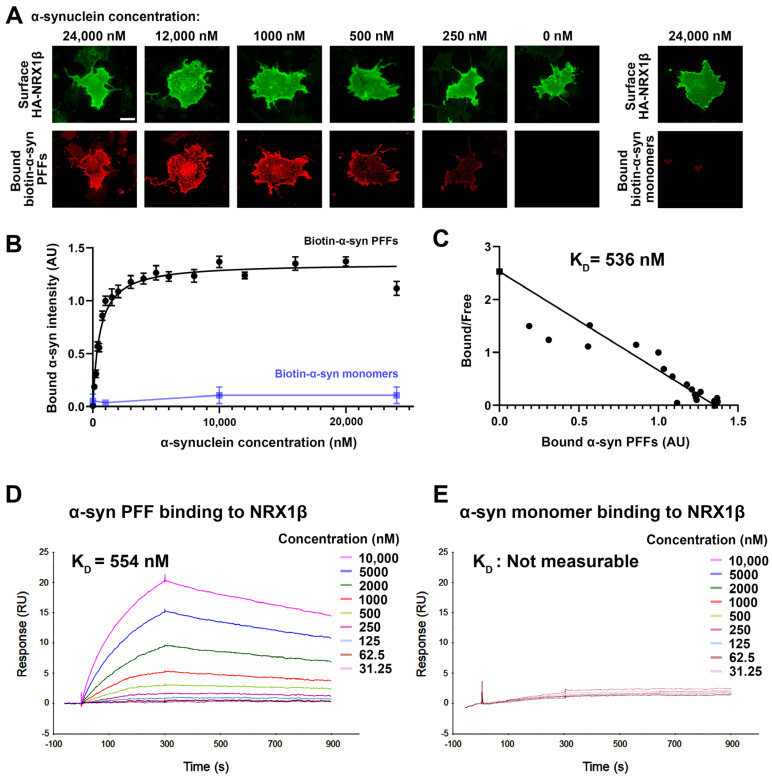
α-syn PFFs, but not α-syn monomers, bind directly to NRX1β. (A) Representative images of cell surface binding assays showing COS-7 cells expressing extracellular HA-tagged neurexin 1β (HA-NRX1β) treated with biotin-conjugated α-syn PFFs at the indicated concentrations (0–24,000 nM monomer equivalent) or treated with 24,000 nM biotin-α-syn monomers. The surface HA (green) was immunolabelled to verify the expression of these constructs on the COS-7 cell surface. Scale bar: 30 μm. (B) Saturable binding of biotin-α-syn PFFs to COS-7 cells expressing HA-NRX1β. Biotin-α-syn PFFs display a saturable binding curve, whereas biotin-α-syn monomers show no binding, even at high concentrations. Data are presented as mean ± SEM (n = 30 cells for each plot from three independent experiments). (C) A Scatchard plot of the biotin-α-syn PFF binding data from (B) indicates a KD of 536 nM monomer equivalent. (D,E) Representative sensorgrams from surface plasmon resonance (SPR) analysis for the binding of untagged α-syn PFFs (D) or untagged α-syn monomers (E) to the purified recombinant NRX1β ectodomain fused to human immunoglobulin Fc (NRX1β-Fc) immobilized on the sensor chip. The KD value of α-syn PFFs is 554 nM. The KD value of α-syn monomers could not be determined, and the sensorgrams indicated no significant binding of α-syn monomers to immobilized NRX1β.