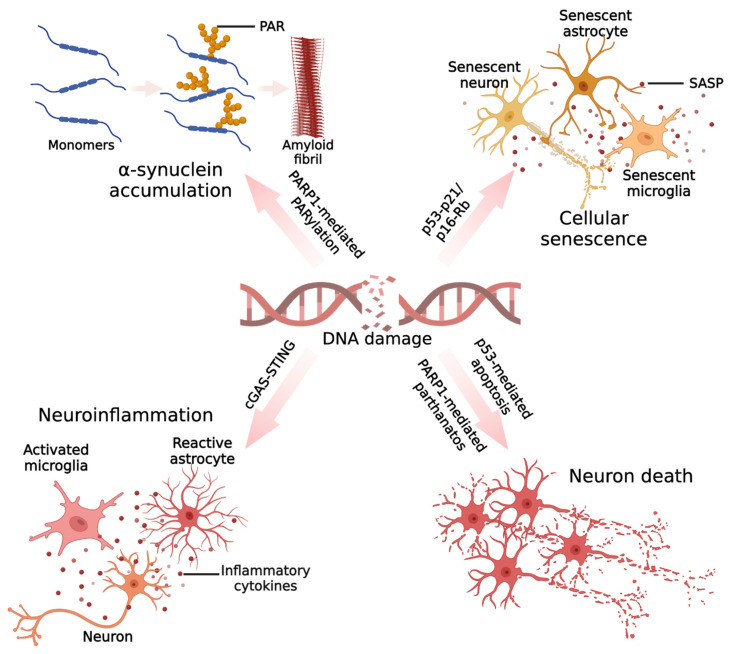
Figure 2.
Mechanisms by which DNA damage could promote neurodegeneration in PD. In PD, the accumulated DNA damage leads to excessive activation of PARP1, and activated PARP1 can synthesize a large number of PAR chains. PAR is able to interact with the N-terminal portion of α-synuclein and promotes the fibrillation of α-synuclein monomers. Cellular senescence phenotypes have been reported in PD models, including senescent neurons, senescent microglia, and senescent astrocytes. In neurons, α-synuclein can induce DNA damage and upregulate the senescence markers p53 and p21. In microglia and astrocytes, pathogenic α-synuclein leads to the upregulation of P21 expression. In addition, the neurotoxin paraquat can cause astrocyte senescence phenotypes in vivo and in vitro, including DNA damage accumulation, p16 upregulation, and senescence-associated secretory phenotypes (SASPs). DNA damage has recently been found to induce inflammatory responses in several types of neuronal cells, including microglia, astrocytes, and neurons. The innate immune response induced by the cGAS–STING pathway plays a major role. Evidence for DNA damage-induced neuroinflammation in PD has been demonstrated in microglia. α-synuclein can attack the genomic DNA of microglia, which in turn activates the STING-dependent inflammatory response, leading to a massive release of inflammatory factors. The oxidative stress induced by inflammatory factors may further lead to DNA damage in other cells, contributing to the continued activation of the DNA damage response and causing a vicious cycle between DNA damage and inflammation. Lastly, DNA damage is thought to be an important cause of dopaminergic neuron death. Specifically, on one hand, DNA damage in PD models can activate a P53-dependent apoptotic program, and on the other hand, persistent DNA damage-induced hyperactivation of PARP1 can trigger parthanatos in neurons. (Created with BioRender.com).