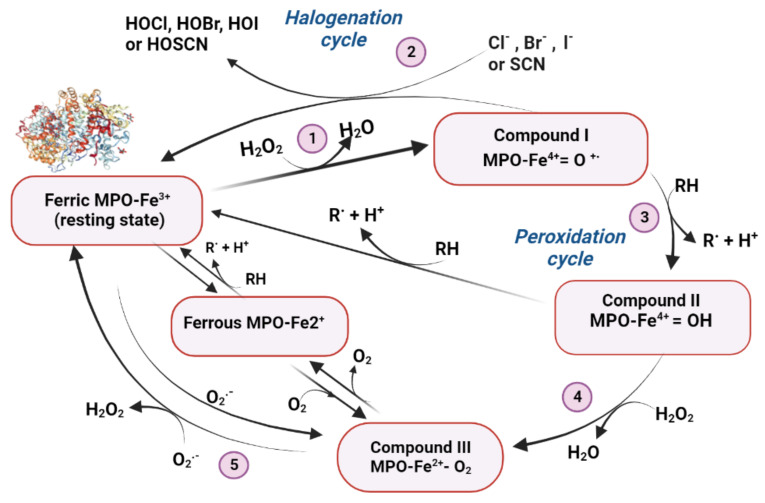
Figure 3.
The myeloperoxidase (MPO) catalytic cycle. Ferric myeloperoxidase (MPO-Fe3+ in its resting state) reacts reversibly with hydrogen peroxide by oxidation of the heme group and formation of the ferryl-p cation radical intermediate compound I (1). This intermediate can oxidize halides and the pseudo-halide thiocyanate to the corresponding hypohalous acids, while being readily reduced to MPO-Fe3+ (2). Compound I also oxidizes multiple organic substrates (RH) to free radicals (R.) in the classical peroxidase cycle which involves two sequential one-electron reduction steps, generating compounds II (3) and III (4). Peroxidation substrates include tyrosine estradiol, serotonin, norepinephrine, ascorbate, urate, etc. Superoxide can also be the substrate for compound I and compound II in these stages. Ferric myeloperoxidase can be reduced to its ferrous form MPO-Fe2+, which binds to O2, forming the oxymyeloperoxidase, or compound III (MPO-Fe2+O2). Superoxide also provides the substrate for the fast conversion of the ferric enzyme to form compound III (5). Ferrous myeloperoxidase and compound III can display catalytic action by reductively activating quinones (adapted from Podrez et al., [173]).