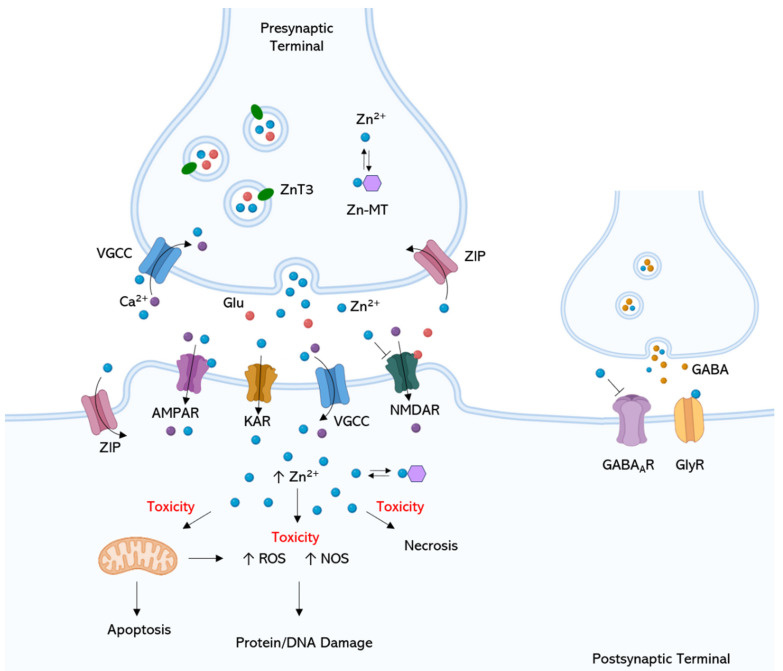
Figure 2.
Physiological and pathological neuromodulation of Zn-containing pre- and postsynaptic neurons. Most vesicular Zn co-localizes with glutamate in subsets of glutamatergic zinc-enriched neurons, and it is also contained in the synaptic vesicles of subpopulations of glycinergic and GABAergic neurons. Zn2+-level regulation between cellular compartments, organelles, and extracellular space is ensured by ZIP and ZnT protein families, and by metallothioneins (MTs), which buffer cytoplasmic Zn2+, functioning as a temporary store for cellular Zn2+. In the presynaptic terminals, Zn2+ is transported into presynaptic vesicles by the Zn transporter ZnT3. During synaptic transmission, free Zn is released in the synaptic cleft, where it may be recycled back into the presynaptic boutons by ZIP/Zn2+ transporters or modulate excitatory (NMDA, AMPA) and inhibitory (GABA, glycine) amino acid receptors of the postsynaptic terminal; Zn can inhibit GABAAR and NMDAR, and potentiate/inhibit AMPAR and GlyR at low/high concentrations, respectively. Extracellular Zn can also alter the excitability of neurons through effects on voltage-gated ion channels (e.g., VGCC), affecting ions’ influx and neurotransmitter release. Ion channels and AMPAR/KAR allow synaptically released Zn to enter presynaptic and postsynaptic neurons to modulate intracellular Zn signaling functions. Excessive Zn2+ accumulation inside postsynaptic cells, as per excitotoxic stimulation, can lead to a series of toxic effects involving mitochondrial dysfunction and ROS/NOS production, eventually leading to oxidative damage to proteins and DNA, neuronal apoptosis, and/or necrosis. Glu, glutamate; GABAAR, GABA A receptor; NMDAR, NMDA receptor; AMPAR, AMPA receptor; GlyR, glycine receptor; VGCC, voltage-gated calcium channel; KAR, kainate receptor.