Fig. 1.
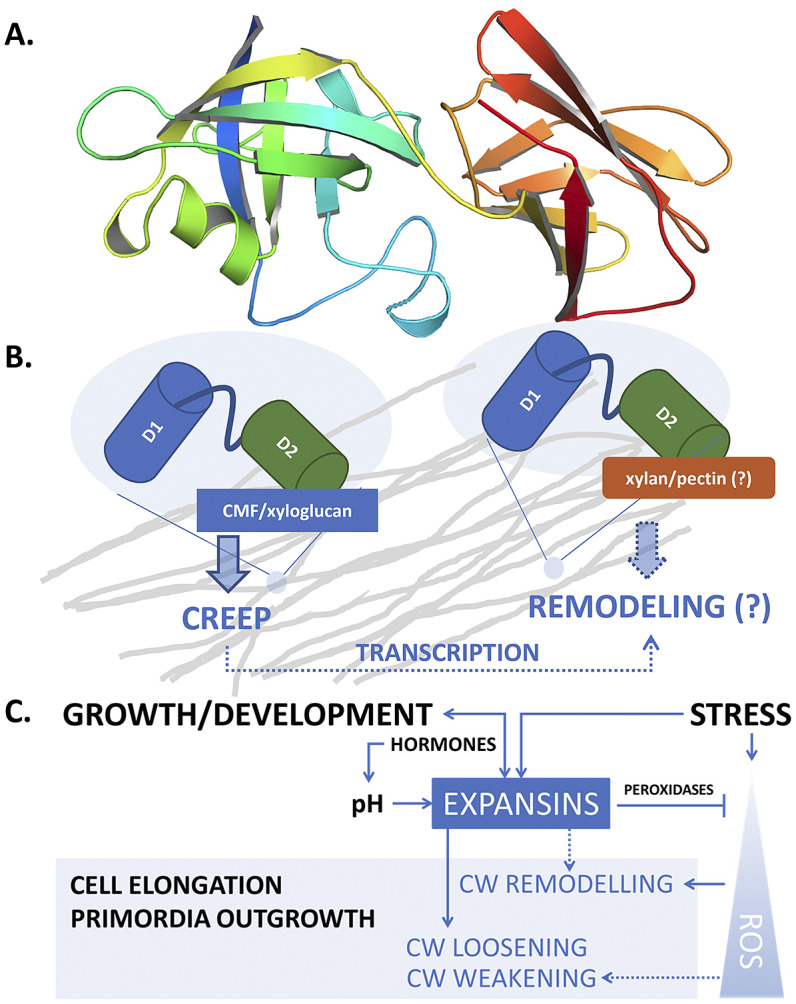
(a) Structure of AtEXPA1 determined by the AlphaFold algorithm. N-terminal six-stranded double-psi (ω) β-barrel D1 domain related to family 45 glycoside hydrolases (GH45) (green/blue, left) and C-terminal β-sandwich fold D2 domain related to group-2 grass pollen allergens resembling the carbohydrate binding module (CBM) family 63 (red/orange, right); the unstructured signal peptide is not shown. (b) Upon binding the load-bearing cellulose microfibril (CMF) network laterally interconnected with possible xyloglucan contribution (grey), expansins induce CW expansion via CW creep. By interfering with CW remodelling enzymes via binding to xylan and/or pectin or through transcriptional feedback regulations in a response to changed CW biomechanics, expansins might contribute to CW remodelling, too. (c) Expansin expression and localization is regulated during plant development, ensuring expansin action in a manner that is specific to their dose and the particular developmental context. Conversely, expansin action on CW biomechanics affects plant development and growth responses by regulating cell elongation and/or primordia specification/outgrowth. Expansins are activated in response to various stresses associated with ROS production. Expansin expression might be mediated by developmental- and stress-regulated hormone production, controlling expansin activity also via spatial-specific CW acidification. Expansins could mitigate ROS effects by upregulating CW peroxidases. In turn, ROS also contribute to the regulation of CW biomechanical properties. While short-term or low-level ROS production leads to growth inhibition by inducing crosslinking of CW components, high ROS levels/long-term ROS production leads to OH°-radical formation that was hypothesised to allow restoration of cell expansion via polymer cleavage, leading to CW weakening. See the main text for a more detailed description.
