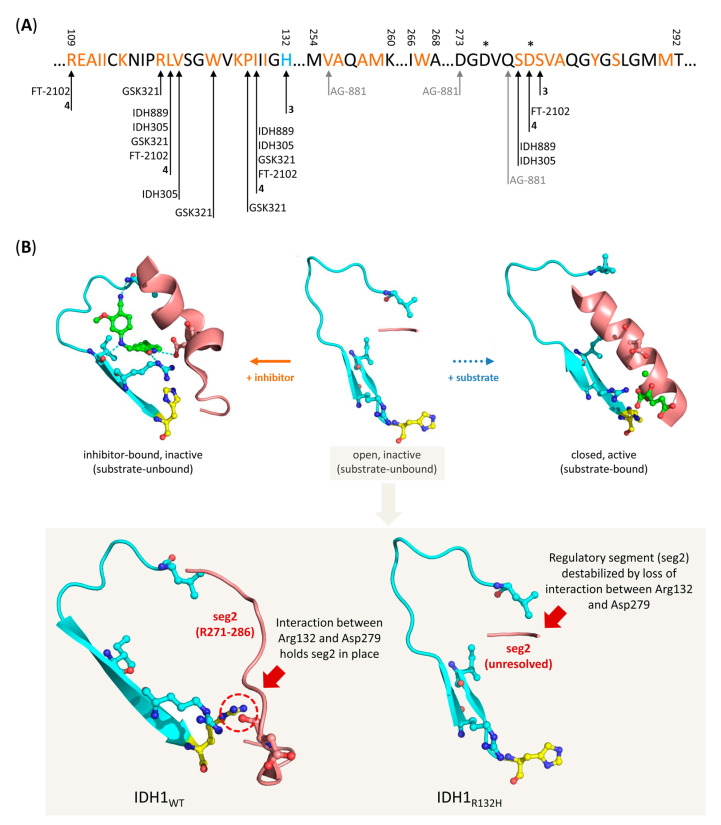
Figure 5.
Overview of residues involved in binding of mIDH-selective inhibitors and proposed mechanism for selective inhibition of IDH1R132H. (A) Amino acid sequence of the allosteric pocket near the substrate binding site of IDH1R132H targeted by most mIDH1-selective inhibitors, and overview of residues involved in inhibitor binding. The mutated histidine residue and residues forming the allosteric pocket are highlighted in blue and orange, respectively, while residues that directly interact with different inhibitors or catalytic Mg2+ ions are indicated by arrows or stars, respectively. Note that the pan-mIDH1/2 inhibitor AG-881 binds to an alternative allosteric pocket at the dimer interface but directly interacts with one of the residues (Val255) lining the allosteric pocket targeted by mIDH1-selective inhibitors. (B) Cartoon representation of the allosteric pocket (indicated in turquoise) and regulatory segment 2 (indicated in red) in IDH1R132H as observed in the crystal structure of the open, inactive (middle, PDB: 3MAR), closed, active (right, PDB: 3INM) or an inhibitor-bound, inactive (left, PDB: 6o2y) conformation. The inhibitor (compound 4, for details, see Section 7.4) is shown in green, while the mutated residue (His132) is shown in yellow. Note that the regulatory segment is destabilized and (due to conformational motions) unresolved in the crystal structure of the inactive conformation (middle), so that inhibitor-binding to the allosteric pocket can lock the enzyme in a quasi-open, inactive conformation (left), while it assumes a long α-helix structure that prevents access to the allosteric pocket in the active conformation (right). Inset: Comparison of the allosteric pocket and regulatory segment 2 in the inactive conformations of IDH1WT (left, PDB: 1T09) and IDH1R132H (right, PDB: 3MAR). Note that interaction between Arg132 and Asp279 in the wildtype enzyme restricts the conformational flexibility of regulatory segment 2, which may limit access to the allosteric pocket in inactive wildtype enzymes.