Figure 1. Proportion of patients who underwent completion lymph node dissection and received adjuvant systemic therapy before and after DeCOG-SLT and MSLT-2 publication and region-specific regulatory approvals of adjuvant immunotherapies and BRAF/MEK inhibitor therapy.
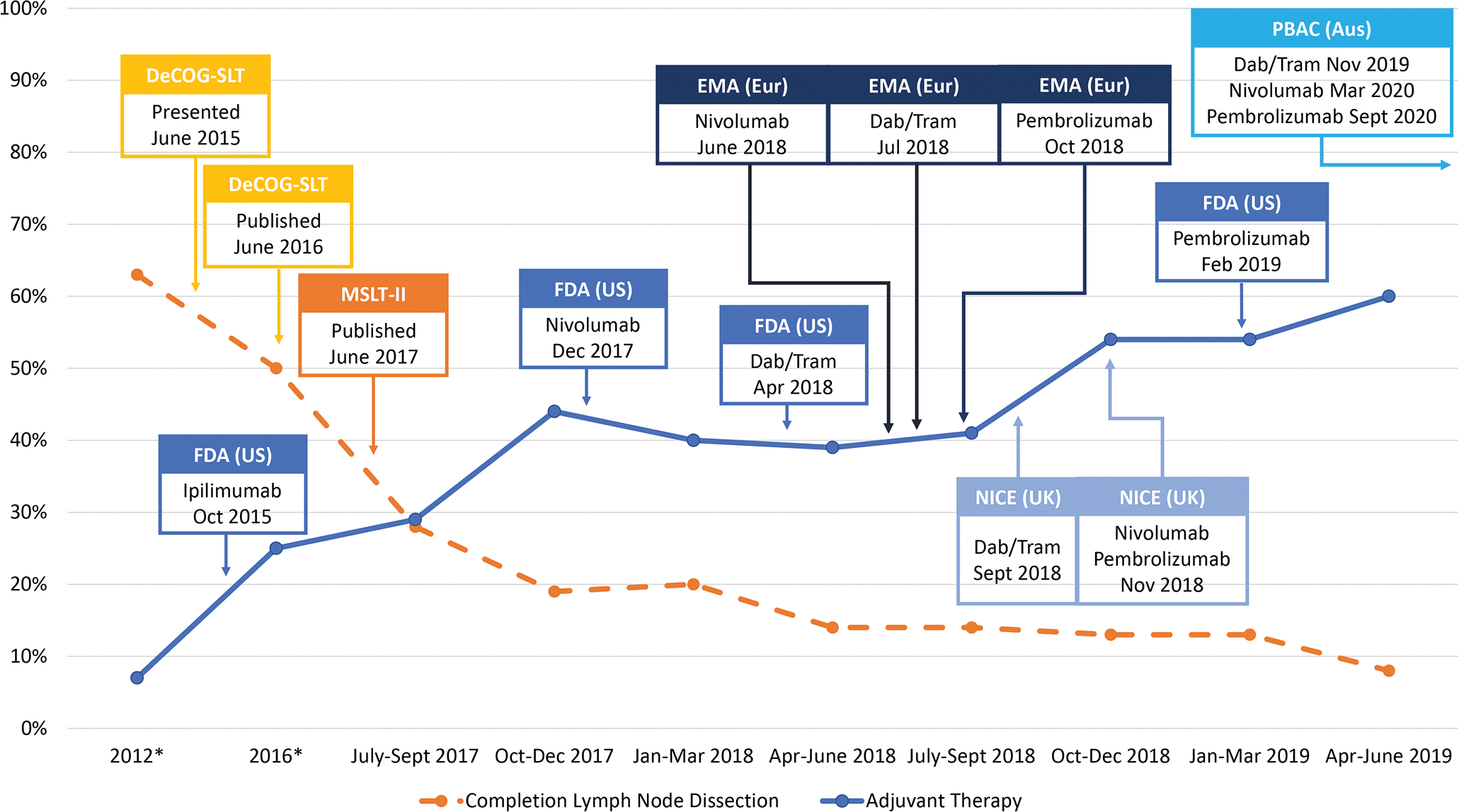
*Historical rates of CLND for years 2012 and 2016 and of adjuvant systemic therapy for 2016 for resected stage III melanoma were obtained from the National Cancer Database; historical rate of adjuvant systemic therapy from 2012 derived from MSLT-II and DeCOG-SLT publications
Abbreviations: DeCOG-SLT = German Dermatologic Oncology Group Trial; MSLT-II = Second Multicenter Selective Lymphadenectomy Trial; FDA = United States Food and Drug Administration; EMA = European Medicines Agency (Europe); NICE = National Institute for Healthcare Excellence (United Kingdom); PBAC = Pharmaceutical Benefits Advisory Committee (Australia); Dab/tram = dabrafenib/trametinib
