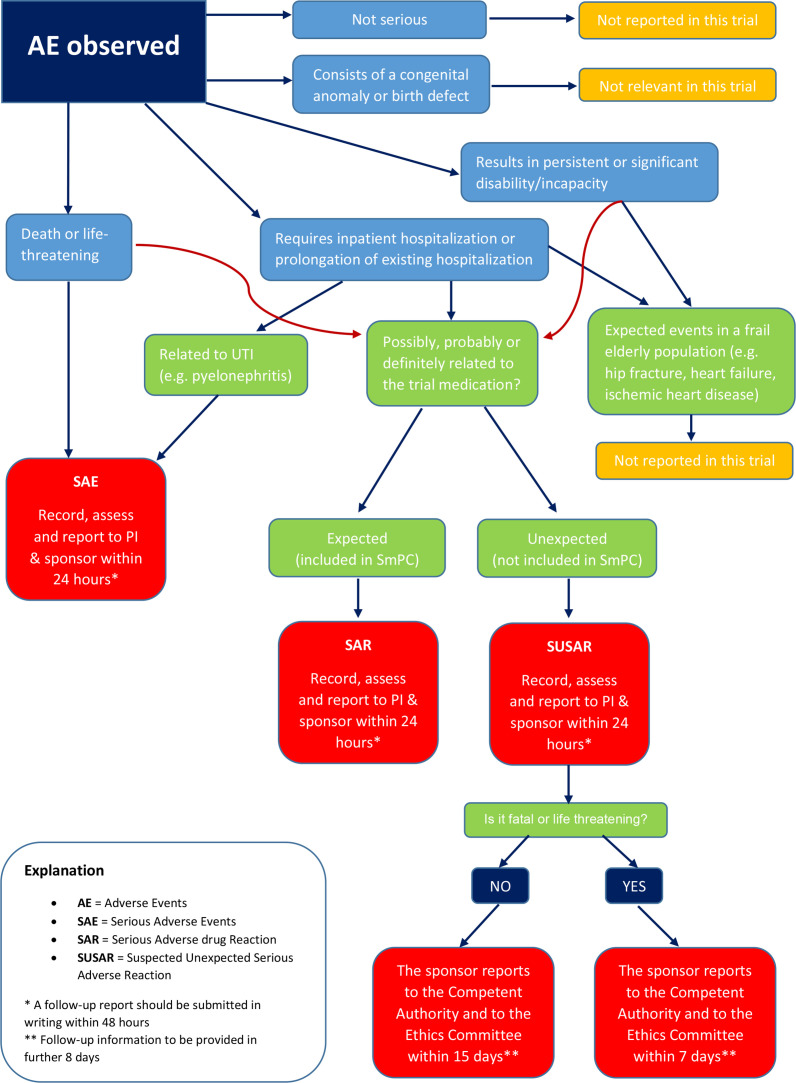
Figure 1.
Safety reporting flow chart for the ImpresU clinical trial. *A follow-up report should be submitted in writing within 48 hours. **Follow-up information to be provided in further 8 days. AE, adverse event; PI, principal investigator; SAE, serious adverse event; SAR, serious adverse drug reaction; SUSAR, suspected unexpected serious adverse reaction; UTI, urinary tract infection.