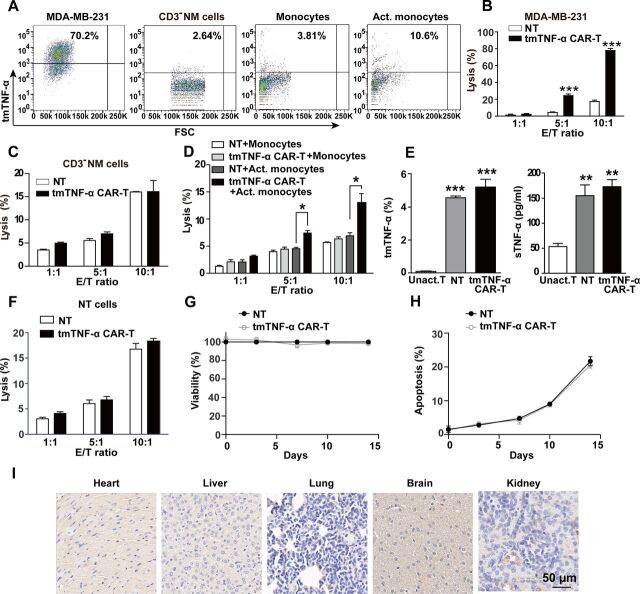
Figure 4.
Estimation for the safety of tmTNF-α CAR-T therapy. (A) tmTNF-α expression in MDA-MB-231, CD3-negative non-adherent mononuclear cells (CD3− NM cells), monocytes and Act. monocytes with conditioned medium (supernatants from 12 hours’ culture of MDA-MB-231 cells) was detected by flow cytometry. The cytotoxicity of tmTNF-α CAR-T cells toward MDA-MB-231 (B), CD3− NM cells (C) and monocytes (D) at different E:T ratios was determined by Cam-release assay. (E) tmTNF-α expression and sTNF-α release by Unact. T cells, NT and CAR-T cells were detected by flow cytometry and ELISA, respectively. (F) Lysis of NT cells (as target cells) by CAR-T and NT cells at different E:T ratios. (G, H) tmTNF-α CAR-T and NT cells were cultured in medium supplemented with IL-2 (50 IU/mL) in plates coated with anti-CD3/anti-CD28. Viability and apoptosis of NT and CAR-T cells were analyzed at indicated time points by CCK8 and annexin V/PI, respectively. (I) Representative immunohistochemical images of tmTNF-α staining for tissue sections of heart, liver, lung, brain and kidney (magnification ×200) from NOD/SCID mice. The quantitative data represent means±SEM of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 versus NT, except E (vs Unact. T cells). Act., activated; CAR-T, chimeric antigen receptor engineered-T; E:T, effector:target; IL, interleukin; NT, non-transduced T; sTNF-α, secreted tumor necrosis factor alpha; tmTNF-α, transmembrane tumor necrosis factor alpha; Unact., unactivated