Abstract
Objective
Anterior temporal lobectomy (ATL) for medication‐resistant localized epilepsy results in ablation or reduction of seizures for most patients. However, some individuals who attain an initial extended period of postsurgical seizure freedom will experience a later seizure recurrence. In this study, we examined the prevalence and some risk factors for late recurrence in an ATL cohort with extensive regular follow‐up.
Methods
Included were 449 patients who underwent ATL at Austin Health, Australia, from 1978 to 2008. Postsurgical follow‐up was undertaken 2–3 yearly. Seizure recurrence was tested using Kaplan–Meier analysis, log‐rank test, and Cox regression. Late recurrence was qualified as a first disabling seizure >2 years postsurgery. We examined risks within the ATL cohort according to broad pathology groups and tested whether late recurrence differed for the ATL cohort compared to patients who had resections outside the temporal lobe (n = 98).
Results
Median post‐ATL follow‐up was 22 years (range = .1–38.6), 6% were lost to follow‐up, and 12% had died. Probabilities for remaining completely seizure‐free after surgery were 51% (95% confidence interval [CI] = 53–63) at 2 postoperative years, 36% (95% CI = 32–41) at 10 years, 32% (95% CI = 27–36) at 20 years, and 30% (95% CI = 25–34) at 25 years. Recurrences were reported up to 23 years postoperatively. Late seizures occurred in all major ATL pathology groups, with increased risk in the "normal" and "distant lesion" groups (p ≤ .03). Comparison between the ATL cohort and patients who underwent extratemporal resection demonstrated similar patterns of late recurrence (p = .74).
Significance
Some first recurrences were very late, reported decades after ATL. Late recurrences were not unique to any broad ATL pathology group and did not differ according to whether resections were ATL or extratemporal. Reports of these events by patients with residual pathology suggest that potentially epileptogenic abnormalities outside the area of resection may be implicated as one of several possible underlying mechanisms.
Keywords: breakthrough seizures, epilepsy surgery, histopathology, long‐term, outcome, resection
Key Points.
We examined late recurrence in an ATL cohort with extensive regular postsurgical follow‐up.
Postoperatively, 51% of patients were seizure‐free at 2 postoperative years and 30% were seizure‐free by 25 years.
First seizure recurrences were reported up to 23 years after surgery.
Late seizures were reported in all broad ATL pathology groups.
The "normal" and "distant lesion" pathologies demonstrated increased risk, implicating residual abnormality as a cause of late recurrence.
1. INTRODUCTION
Temporal lobe resection is an effective treatment for well‐localized focal epilepsy that has not responded to antiseizure medication (ASM). Studies have demonstrated that most individuals who undergo resection of their seizure focus attain seizure freedom or significant reduction in seizures after surgery. 1 Unfortunately, for some individuals, the seizure freedom experienced in the first few years after surgery may not be maintained, with late seizure recurrence reported in a number of studies. 2 , 3 , 4 , 5 , 6 This outcome is potentially devasting, as many individuals have made a new life 7 and may believe surgery has cured their epilepsy. Although individuals live with the outcomes of surgery for the rest of their lives, little is understood about late recurrence due to the paucity of large cohorts with extended follow‐up time.
We have previously described long‐term seizure outcomes for the postsurgical anterior temporal lobectomy (ATL) cohorts in the Austin Health Comprehensive Epilepsy Program (CEP), Melbourne, Australia. 3 , 8 , 9 Since that time, more than a decade of additional follow‐up has been undertaken and more than half of these patients are now at least 20 years postoperative. Here, we utilize these additional data from this large cohort with regular follow‐up to examine the characteristics of late postoperative seizure recurrence.
We asked:
What is the frequency of late seizure recurrence after ATL in patients with extensive postsurgical follow‐up?
How late in the postoperative follow‐up do first recurrences present?
Within the ATL cohort, does the risk of late recurrence differ across major preoperative pathology groups?
Do ATLs undertaken in the early years of the cohort (i.e., before advanced imaging) have higher risk of late recurrence?
Does the frequency of late recurrence after ATL differ compared to resective surgery undertaken outside the temporal lobe (i.e., extratemporal surgery)?
These data will offer insights into very long‐term outcomes, contribute to knowledge of risk factors for late seizure recurrence, and facilitate ongoing management and counseling of pre‐ and postsurgical patients.
2. MATERIALS AND METHODS
Included in this study were patients who underwent standard ATLs at the Austin Health CEP from 1978 to 2008. The 2008 cutoff point means long‐term postoperative follow‐up was available. Three hundred twenty‐five patients who had ATL in the years 1978–1998 had been the subject of previous studies, 3 , 10 and we added an additional 124 individuals who had surgery after 1998. All ATLs in this time frame were included apart from patients who had their first seizure surgery at another hospital and 27 ATL cases operated before 1987 with insufficient information to establish preoperative pathology. 3
A total of 449 predominantly adult ATL patients met the criteria for this study. Preoperative evaluation and surgical procedures have been described previously, 3 , 11 and surgical details are also included in the supplementary information. Patients typically remain on ASM for a minimum of 2 years postsurgery, and any taper/discontinuation after that time is tailored to the individual. Patients are discharged to their usual neurological care after 2 postoperative years with the CEP long‐term follow‐up program in place.
Postsurgical follow‐up is systematic and ongoing. 3 Trained CEP staff routinely seek follow‐up information at least every 2–3 years. This may take the form of correspondence from the treating doctor, but commonly involves telephone contact with the patient or their family. In the latter cases, a brief and informal semistructured telephone interview is conducted covering the period since the patient was last contacted. This is expanded according to the reported circumstances and requirements (i.e., if the patient has queries, their seizure condition has changed, or a potential seizure recurrence is reported), and occasionally witness reports or information from treating doctors or hospitals may be requested. In some cases, reattendance at the CEP or follow‐up with their treating doctor is recommended.
Rigorous and repeated efforts are made to locate individuals who are lost to follow‐up, including the use of public‐record electoral rolls. Commonly, if a patient dies, this is reported to the program by family or care providers. However, if an individual has been lost for some time, we utilize public resources such as death notices to identify those who are deceased.
2.1. Postoperative seizure outcome and late recurrence
All patients were coded as being seizure‐free or not seizure‐free from the time of surgery. All disabling seizures (e.g., focal unaware [previously coded as complex partial] ± convulsive seizures) were counted. Individuals who experienced events with no impairment (auras/focal aware/nondisabling simple partial seizures) were coded as seizure‐free. 3 Those who were deceased or lost to follow‐up contributed data up to their last follow‐up date. Patients who had a further surgical procedure contributed data up to the date of their second surgery.
Late seizure recurrence was defined as a first postoperative seizure >2 years after surgery. 3 , 11
The frequency and timing of late seizures were investigated using Kaplan–Meier survival curves to plot recurrence from the time of surgery over the extent of follow‐up.
We also undertook three comparisons of late recurrence:
According to major ATL preoperative pathology groups
To assess risk of late recurrence within the ATL cohort according to preoperative pathology, the patients were sorted into five "best evidence pathology" groups. This process was undertaken for most patients previously in McIntosh et al., and is detailed in that study. 3 Briefly, review of the most recent histopathology and magnetic resonance imaging (MRI) information available was undertaken and a decision was made as to the "best evidence" preoperative pathology. For this current study, any additional information after that review was incorporated.
"Best evidence pathology" groups 3 comprised: (1) foreign tissue lesions (FTLs; n = 56; vascular malformations [VMs], tumors, dysembryoplastic neuroepithelial tumors [DNETs], nonneoplastic cystic lesions); (2) hippocampal sclerosis (HS; n = 273); (3) "other" focal temporal lobe lesions (dysplasia and other lesions large enough to be seen macroscopically such as posttraumatic gliosis; n = 29); (4) "normal" temporal lobe (a general term that includes histological abnormalities that were mild and generally diffuse or minor global atrophy on MRI; n = 54); and (5) distant‐lesion cases (n = 37), so called due to the presence of potentially epileptogenic lesion(s) outside the area of ATL resection and not targeted for surgery (heterotopia, tumors, obvious dysplastic lesions, contralateral HS in cases with bilateral HS preoperatively). Some distant lesions were known prior to surgery and others were identified with later review and improved MRI. 3 Lesions/abnormalities with coexistent HS were coded into the pertinent lesion/abnormality group (e.g., FTLs with HS was coded in the FTL group). 3
-
ii
According to year of ATL surgery
To assess whether ATLs performed in the early years of the cohort had a higher frequency of late recurrence, patients were grouped into four groups according to year of surgery. These were designed to reflect the availability of preoperative imaging investigations at Austin Health. Groups were: 1978–1986 (no MRI), 1987–1992 (early MRI), and 1993–2000 and 2001–2008 (MRI, single photon emission computed tomography, and positron emission tomography in the latter two groups). 3
-
iii
ATL versus extratemporal resection
To test whether individuals who underwent ATL had an increased risk of late recurrence compared to those who had resections outside the temporal lobe, ATL outcome was compared to the outcome after extratemporal (ET) resection at Austin Health. Ninety‐eight patients who underwent ET surgery between 1991 and 2008 met the inclusion criteria for this study and were included. Eighty‐one of these (1991–2004) were described in a previous publication, 10 and their resection and pathology information is detailed in that paper. The follow‐up procedure was the same for both cohorts. For a direct comparison between the two cohorts, only ATL patients who had surgery in the same time frame as the ET cohort were included in this analysis.
In addition to the analyses above, we described the characteristics of late seizure recurrence among the ATL cohort, including medication status at the time of recurrence. Seizures were designated ASM withdrawal seizures when they occurred within 7 days of having stopped ASM, a missed dose, or in association with a severe gastrointestinal upset. 3 We also described the first late postoperative seizure type (convulsive/nonconvulsive), and whether the first recurrence was typical or not typical of the presurgery seizures. Seizure status after recurrence will be examined in detail in a future paper and is not included here.
2.2. Data retrieval
Clinical information including follow‐up data were prospectively recorded in CEP and hospital records as part of routine care. Data for this retrospective study were collected via audit of these records. Variables were collected and coded blinded to outcome.
2.3. Statistical analyses
Statistical testing comprised Kaplan–Meier survival analysis examining the association of late seizure recurrence with pathology, era of surgery, and surgery type. Statistical significance was tested using the log‐rank test. For comparison of preoperative pathology groups, univariate Cox regression also enabled calculation of the hazard ratio for each pathology subgroup. Results were statistically significant at the 5% level (two‐sided).
In some cases, the specific dates for seizure recurrence were unknown. In these cases, we estimated dates using the midpoint between two known dates. 3
This study was approved by the Austin Health Human Research Ethics Committee.
3. RESULTS
Of the 449 individuals who underwent ATL, 210 (47%) were male. Median age at surgery for ATL was 31 years (interquartile range [IQR] = 22–40, range = 6–66 years). Fifty‐eight (13%) were <18 years of age at surgery. Median age at last follow‐up was 49.2 years (95% confidence interval [CI] = 41.6–58.2 years, range = 21.1–74.1 years).
Median postsurgical follow‐up was 22 years (IQR = 14–27, range = .1–38.6 years). Ninety‐eight percent of patients had >2 years of follow‐up, 88% had >10 years, and 57% had >20 years of postoperative follow‐up. All of the eight patients with <2 years of postoperative follow‐up had died before the 2‐year anniversary. Over the total period of follow‐up, 55 individuals died (12%); cause of death was possible/probable sudden unexpected death in epilepsy (SUDEP), n = 18; suicide, n = 9; seizure‐related accident, n = 1; brain tumor (new or residual), n = 2; other, n = 19; and unknown, n = 6. Forty‐three (78%) of those deceased had experienced seizure recurrence before death. During follow‐up, three patients had refused further contact and 27 (6%) were lost. Median follow‐up before contact ceased was 11.5 years for deceased patients and 15 years for lost patients. All three individuals who refused further contact had >5 years postsurgical follow‐up.
Twenty‐eight patients had repeat resection of the epileptogenic region. All but one patient had experienced disabling seizures postsurgery. The exception was a patient who had increasing auras and a second operation due to recurrence of their tumor. Two additional individuals with recurrent seizures underwent other surgical procedures (one hemispherectomy, one implantation for deep brain stimulation), and one patient had resection of a tumor unrelated to the original surgery.
Twenty‐six percent (n = 117) of patients had ceased ASM by last follow‐up.
3.1. ATL cohort and late seizure recurrence
Of the total 449 ATL patients, 306 (68%) experienced seizure recurrence over the follow‐up time. At 2 postoperative years, 226 patients (51%) were seizure‐free from the date of surgery. This does not include two seizure‐free individuals who died of causes other than epilepsy before the 2‐year anniversary. Of the 226 who were seizure‐free at 2 years, 85 had subsequent late recurrence.
Figure 1 illustrates the Kaplan–Meier probabilities of seizure freedom after ATL. The vertical line at the 2‐year mark delineates earlier seizure recurrence from our definition of late recurrence. Detailed data are presented in Table 1. Most postoperative seizures occurred within the first 12 months postoperatively. Late recurrences were at a comparatively slower pace, mostly within the 2–10‐year period. Very late recurrences after 10 postoperative years were also evident, with seizure‐free probability dropping by a few percent over each 5‐year period persisting to just after 20 postoperative years. The latest recurrence was in the 23rd postsurgical year. The numbers of patients remaining in the analysis (i.e., seizure‐free and with follow‐up) beyond this time were small, with 46 patients at 25 postoperative years, 11 at 30 years, and two at 35 years.
FIGURE 1.
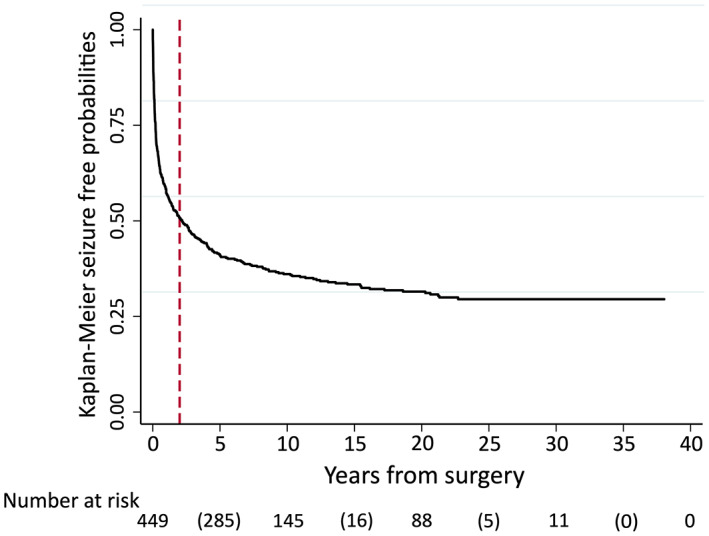
Seizure‐free probabilities after anterior temporal lobectomy. Vertical dotted line indicates 2 postoperative years. Number at risk: numbers without parentheses represent number of patients remaining in analysis at 0, 10, 20, 30, and 40 postoperative years; numbers in parentheses represent number of seizure recurrences during each 10‐year time period.
TABLE 1.
Postsurgery seizure‐free probabilities a for all anterior temporal lobectomy cohort (N = 449) and patients seizure‐free for 2 years (n = 226)
| Postsurgery years | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 year | 2 years | 10 years | 15 years | 20 years | 25 years | |
| All anterior temporal lobectomy cohort | |||||||
| Number at riskb | 449 | (188) 260 | (33) 226 | (64) 145 | (10) 113 | (6) 88 | (5) 46 |
| Seizure‐free, % (95% CI) | 58 (53–63) | 51 (46–55) | 36 (32–41) | 33 (29–38) | 32 (27–36) | 30 (25–34) | |
| Seizure‐free for 2 years after surgery | |||||||
| Seizure‐free % (95%CI) | 100 | 100 | 71 (65–77) | 66 (59–72) | 62 (55–68) | 58 (51–65) | |
Abbreviation: CI, confidence interval.
Kaplan–Meier survival analysis.
bNo parentheses = number of patients remaining in analysis at each time point. Parentheses = number of seizure recurrences during each time period (e.g., 64 recurrences from 2 to 10 years).
3.2. Comparison of late recurrence in major ATL preoperative pathology groups
Figure 2 demonstrates Kaplan–Meier estimates for each of the ATL pathology groups, with additional data in supplementary Table S1. Seizure‐free probabilities are highest for the FTL group. Compared to those with FTLs, seizure‐free probabilities for patients with HS were lower (p = .005). The "normal," "other," and distant‐lesion groups were roughly clustered, with significantly lower seizure freedom than either the FTL or HS groups for all comparisons (all p < .001) except HS versus the "other" group (p = .1).
FIGURE 2.
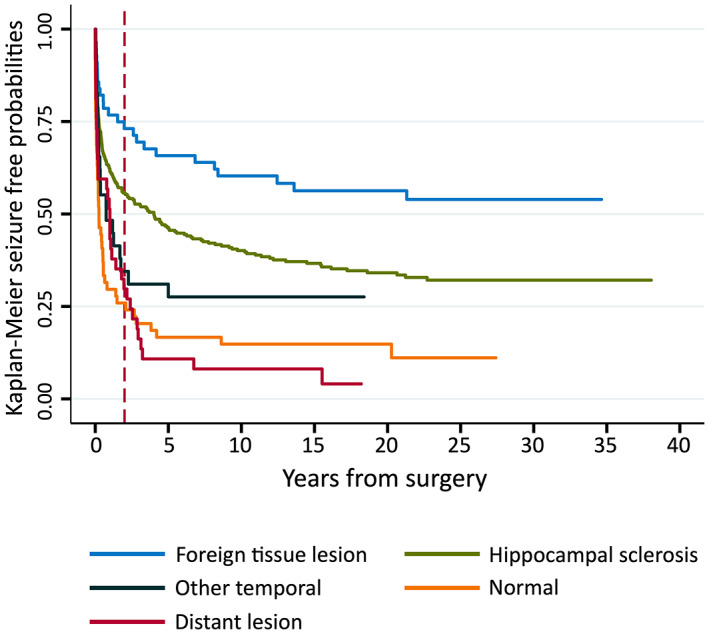
Seizure‐free probabilities by anterior temporal lobectomy "best evidence" pathology group. Vertical dotted line indicates 2 postoperative years. See Supplementary Data Table S1 for details.
All pathology groups demonstrated episodes of late seizure recurrence, as evident in Figure 2. Supplementary data Figure S1 illustrates the same outcome data but exclusively for patients who were completely seizure‐free for the first 2 years postsurgery.
Univariate Cox regression results comprising only those patients who were seizure‐free at 2 postoperative years (n = 226) indicate that when compared to the FTL group (n = 40), the "normal" (n = 14) and distant‐lesion (n = 11) groups had higher late recurrence (compared to FTL: "normal" hazard ratio [HR] = 2.8, 95% CI = 1.1–7.5, p = .03 and distant‐lesion HR = 8.4, 95% CI = 3.4–21, p < .001). When compared to the FTL group, those with HS (n = 151) and "other" pathology (n = 10) were not significantly different (HS HR = 1.7, 95% CI = .9–3.5, p = .1 and "other" HR = 1.1, 95% CI = .2–5.1, p = .9).
3.3. Comparison of late recurrence by year of ATL
Late seizure recurrences occurred in all four groups denoting year of surgery, spanning 1978–1986 (Group 1) to 2001–2008 (Group 4). The earliest group (1978–1986) was not associated with increased risk (log‐rank test p = .9). Supplementary Figure S2 illustrates very similar survival curves for the four groups.
3.4. Comparison of ATL versus ET resection
For direct comparison between the ATL and ET cohort, we included only ATL patients who underwent surgery over the same time as the ET cohort (1991–2008), comprising 301 ATL patients. Median follow‐ups for the ET cohort (17.2 years, IQR = 12–21, range = .02–27) and the ATL subset (18 years, IQR = 12–23, range = .01–27) were very similar.
In the ET cohort, there were 98 individuals with a median age at surgery of 28 years (IQR = 21–38, range = 4–61). Nineteen (19%) were <18 years of age at surgery. ET pathology comprised lesions (tumor, DNET, VM, nonneoplastic cystic lesion), n = 19; acquired insult, n = 17; focal cortical dysplasia, n = 53; inflammation, n = 1; nonspecific findings, n = 8. Forty‐five percent of ET surgery patients (n = 44) were male. Twelve were deceased (12%) (possible/probable SUDEP, n = 4; suicide, n = 2; brain tumor, n = 2; postoperative, n = 1; other, n = 3). One patient had refused further contact, and one was lost to follow‐up. Ten (10%) patients had ceased ASM at last follow‐up. Nineteen underwent a repeat resection.
Figure 3 illustrates probabilities of seizure freedom for the ATL subset and ET group. Both demonstrate initial rapid recurrence, with an early drop substantially more marked in the ET group (log‐rank test p < .001). There is a slow, steady attrition in seizure‐free probabilities after this; late recurrence occurred in 55 of 160 ATL and eight of 22 ET subjects who were seizure‐free for 2 postoperative years. Late survival curves are virtually parallel; the risk of late recurrence did not differ between the two cohorts (log‐rank p = .74).
FIGURE 3.
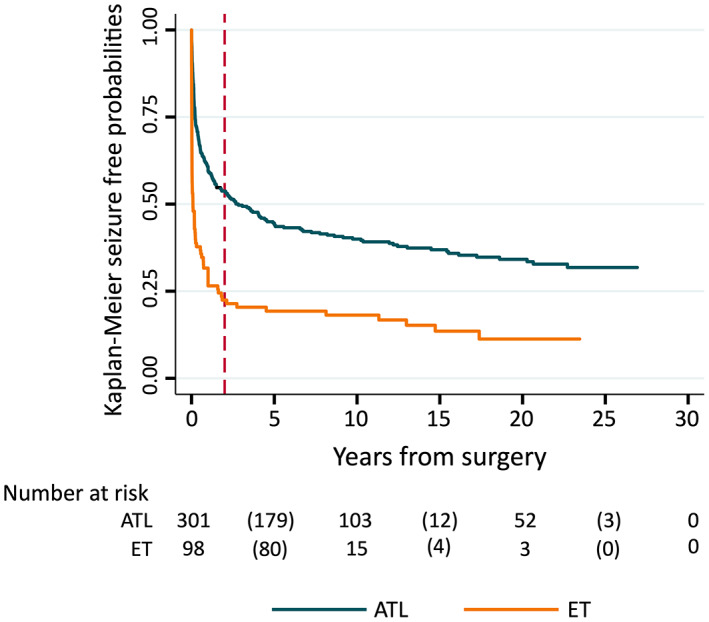
Seizure‐free probabilities for anterior temporal lobectomy (ATL) versus extratemporal resection (ET) surgery. Vertical dotted line indicates 2 postoperative years. Number at risk: numbers without parentheses represent number of patients remaining in analysis at 0, 10, 20, and 30 postoperative years; numbers in parentheses represent number of seizure recurrences during each 10‐year time period.
3.5. Late seizure recurrence characteristics in ATL patients
The characteristics of the first postsurgical seizure are detailed in supplementary information Table S2 according to the timing of recurrence. Convulsive seizures were most common overall. The proportion with nonconvulsive seizure recurrence increased slightly over the years, although numbers are small at extended time periods.
In most cases, it was difficult to clarify whether seizures were typical of the preoperative condition. Convulsive seizures were frequently only recorded as convulsive or tonic–clonic, and any earlier phase of the seizure was either missed or not reported. It was also often difficult to determine whether nonconvulsive seizures were similar to preoperative events, as this aspect was not always specifically noted.
3.6. ASM status at the time of late recurrence
ASM status at the time of first postsurgical seizure (Table 2) shows most of the 85 patients who experienced late recurrence had already ceased ASM (31%) or ASM was stable (32%). Only 5% of recurrences were reported as associated with taper or decreased dose, and 8% were reported with acute drug withdrawal. Acute ASM withdrawal seizures were only reported in the first 2–10 years postsurgery, and recurrences associated with ASM taper were not reported after 10 postoperative years.
TABLE 2.
ASM status at first postoperative seizure in patients with late recurrence, n = 85
| Years postsurgery at recurrence, n (%) | |||||
|---|---|---|---|---|---|
| 2–4.9 | 5–9.9 | 10–14.9 | 15–19.9 | 20+ | |
| ASM withdrawal seizure a | 5 (12) | 2 (10) | 0 | 0 | 0 |
| Seizure during planned ASM taper | 3 (7) | 1 (5) | 0 | 0 | 0 |
| On ASM, no ASM decrease recorded | 12 (28) | 10 (48) | 2 (20) | 1 (1) | 2 (40) |
| ASM ceased <1 year before recurrence | 9 (21) | 0 | 1 (10) | 0 | 1 (20) |
| ASM ceased >1 year before recurrence | 4 (9) | 1 (5) | 3 (30) | 5 (83) | 2 (40) |
| ASM ceased/tapered, relationship with seizure unknown | 2 (5) | 3 (14) | 2 (20) | 0 | 0 |
| ASM status unknown | 7 (19) | 4 (19) | 2 (2) | 0 | 0 |
| Total p | 43 | 21 | 10 | 6 | 5 |
Abbreviation: ASM, antiseizure medication.
Within 7 days of ceasing ASM (missed a dose or stopped with/without taper) or a severe gastrointestinal upset.
3.7. Other potential seizure precipitants
One ATL patient had late recurrence at 18 years postsurgery and was diagnosed 4 months later with a contralateral frontoparietal tumor.
Other circumstances that were reported by patients and mentioned in the medical notes as possibly associated with late seizure recurrence were health‐related (e.g., acute illness, cancer diagnosis and treatment, onset of depression, commencement of medication, childbirth), lifestyle‐related (e.g., sleep deprivation, stress, alcohol), or general physical issues (overexertion, low‐grade "unwell," activity in hot weather). We did not conduct formal analyses using these data, as they were not systematically collected.
4. DISCUSSION
In our cohorts, the great majority of seizures recur in the first postoperative year, a pattern also described in other studies. 1 , 3 , 6 , 10 , 12 , 13 , 14 , 15 , 16 , 17 By 2 postoperative years, the probability of seizure freedom in our ATL cohort was 51% (Figure 1), with the early rapid drop in seizure freedom most obvious for the "normal" and "other" temporal groups, and the distant‐lesion group (Figure 2).
Early studies by Rasmussen 18 described long‐term surgical outcome, noting 13% of "nontumoral" (median follow‐up = 12 years) and 6% of "tumoral" cases (median follow‐up = 8 years) experienced occasional seizures after at least 3 years free of seizures postsurgery. Late recurrences between 5 and 10 years postsurgery have been described in more recent cohorts. 2 , 5 , 6 , 14 , 17 , 19 , 20 There are few detailed data available relevant to the period after 10 postoperative years, although first recurrences after this time have been noted. 2 , 14 , 21 , 22 In our study, the data demonstrate seizure recurrence over several decades of follow‐up. The pattern of attrition slows over time, with probabilities for the ATL cohort as a whole dropping to 36% at 10 years, 33% seizure‐free at 15 years, and 32% at 20 postoperative years. The last seizure recurrence was in the 23rd postoperative year. Although there were no recurrences beyond this time point, this may be a function of smaller numbers at the extreme end of our follow‐up. 3 However, it is also possible that the risk of late recurrence decreases further as time goes on; the question is whether it ever becomes equivalent to the population incident risk.
Late recurrence occurred in all ATL pathology subgroups, indicating this phenomenon was not unique to one group. Neither was it unique to the ATL cohort. Figure 3 illustrates a very similar pattern of late recurrence for those who had ATL compared to the ET cohort, with the difference in risk between these two groups (higher for ET cohort) restricted to the early postsurgical period. Other studies have also reported late seizures in cohorts with a mixture of pathologies and/or surgeries 2 , 14 , 17 , 19 , 23 ; abnormal and/or normal pathology 15 ; lesional and/or nonlesional cases 24 , 25 , 26 , 27 , 28 , 29 ; focal cortical dysplasia 30 ; temporal lobe and "temporal plus" epilepsy 20 , 31 ; and ATL, ET, and amygdalohippocampectomy surgeries. 5 , 6 , 12 , 22 , 32 , 33 These findings favor hypotheses relating to causes of late seizure recurrence that apply broadly across these groups.
We found the ATL distant‐lesion and "normal" subgroups were more likely to experience late recurrence, with the earlier drop in seizure freedom continuing into our nominated late recurrence period of >2 postoperative years (Figure 2). In our study, ATL distant‐lesion cases are defined by the presence of pathology outside the temporal lobe that was not targeted for resection. Although this group is at high risk of early seizure recurrence, that late recurrence is also a feature implicates preexisting/residual epileptogenic structural abnormality as a cause of late seizures. Our findings are concordant with a recent study that compared patients with a variety of pathologies and late recurrence to patients without recurrence, finding that incomplete resection of the abnormality or bilateral lesions were risk factors for late recurrence. 2 Although we had previously reviewed and updated pathology classification in our cohort where possible, 3 the similarity of seizure recurrence patterns between our distant lesion and "normal" groups seen in Figure 2 raises the question of whether some patients with presumed normal imaging actually have an unidentified abnormality outside the area of resection. 3 , 11 Advances in imaging may identify pathology in historical and recent cohorts that was not obvious at the original investigation.
Recurrences may also relate to the activity of a residual seizure focus other than a structural abnormality. Hypotheses include aberrant activity in distributed brain networks involving but not restricted to the region of the resected tissue, 20 , 34 , 35 , 36 , 37 or the effects of genetic variants present throughout the brain. 38 , 39 "Temporal‐lobe plus" epilepsy (TPE), with an epileptogenic zone extending beyond the temporal lobe identified by stereo‐electroencephalography, has been associated with recurrence in HS and nonlesional cases. 20 , 31 It is also possible that new seizure mechanisms evolve over time. In particular, age‐related seizure conditions may present as postsurgical follow‐up extends into decades and patients grow older. 40 Another hypothesis proposes that the postresection gliosis evolves as a site of epileptogenesis: “ripening of the scar” as initially conceptualized by Penfield and Jasper. 41
Also important is the corresponding question of why some patients who are at high risk of postoperative seizures (i.e., with residual pathology or extensive epileptogenic zone) can experience years of seizure freedom before recurrence. Surgery may initially disrupt preexisting seizure networks, 42 but seizure activity could become reestablished over time. Of interest, data from one study of patients with an extensive epileptogenic zone (i.e., TPE) suggest that larger resections incorporating areas of seizure onset outside the temporal lobe may be associated with a longer delay to seizure recurrence. 20
All hypotheses discussed above relate to mechanisms of seizure generation. Another possible cause of delayed recurrence in some high‐risk individuals is disruption of ASM control. Resective surgery may modify drug‐resistant epilepsy to the extent that the condition becomes drug‐responsive. 43 , 44 , 45 Seizures in these patients may be well controlled until a decrease or withdrawal of ASM, 44 although most patients in our cohort were either established on medications or had ceased them at the time of recurrence. Another possibility is that medication requirements increase or change in circumstances of illness or other life events that exert major physiological and/or metabolic stress. This is pertinent to patients controlled on ASM, as well as those who have ceased medication but still require ASM to manage their seizure condition over challenging periods. Of relevance, Coleman et al. 7 previously studied a subgroup of our ATL cohort, reporting potentially stressful events that spanned the approximately 20 of follow‐up. These included pregnancy and childbirth, changes to employment and education, and relationship conflict. Major health conditions may develop over extended follow‐up, as evident in our cohort, where individuals reported new diagnoses of cancer or depression with associated proconvulsant medication.
Some mechanisms postulated above may be time‐dependent. This is represented schematically in Figure 4. Within a postsurgical cohort, the contribution of residual pathology or epileptogenesis and ASM withdrawal to seizure recurrence may feature heavily in the first few postoperative years and then drop off, whereas other mechanisms take longer to manifest. Research into potential time‐dependent causes of recurrence becomes possible as follow‐up data accumulate in surgical cohorts. Although the use of a single postoperative time to define late seizure recurrence (i.e., 1 or 2 postoperative years) is relatively common, the research limitations of this approach are highlighted by the recurrences seen in our data (Figure 1) that occur years beyond our study definition of "late seizures" (as marked in Figure 1 with a line at 2 years). Future researchers may be able to utilize long follow‐up to identify and define several periods of differing postoperative risk.
FIGURE 4.
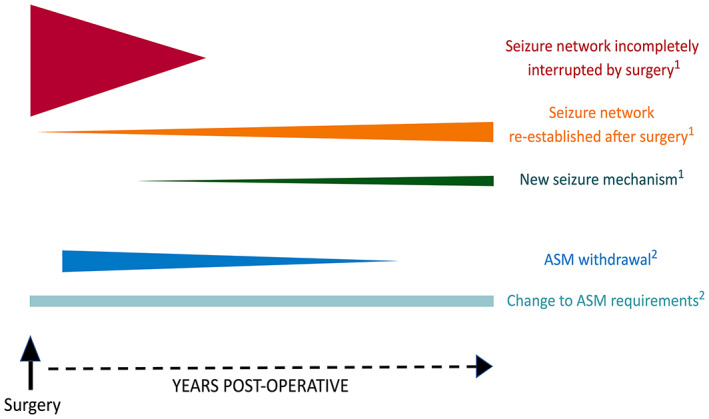
Hypothesized mechanisms of seizure recurrence after epilepsy surgery × postoperative time. Each line reflects a possible mechanism of seizure recurrence after surgery. The position of each line reflects hypothesized postoperative timing of onset, and line height reflects the degree of potential impact. A variety of mechanisms may be responsible for seizure recurrence within a surgical cohort. 1Recurrence due to seizure generation. A seizure focus/network (i.e., due to residual pathology or extensive epileptogenesis) that is incompletely interrupted by surgery is probably associated with a high risk of seizure recurrence in the initial postoperative period but is less likely to be a cause of recurrence in later years. Interruption of the seizure focus/network by surgery may render patients seizure‐free initially, but seizure activity may be reestablished after some years. New seizure mechanisms such as age‐related brain disorders may take time to evolve. 2Recurrence due to disruption of control in antiseizure medication (ASM)‐responsive epilepsy. Attempts at ASM withdrawal or taper usually start after the initial postsurgical period, and our data suggest seizures associated with this factor become less common over time. ASM requirements may change due to stressful social or health events, an issue that is relevant to patients on medications as well as those who previously ceased ASM but require ASM for seizure control during challenging periods.
Our ATL data span many years, including the preimaging era, but we found no difference for risk of recurrence in any of the four groups denoting year of surgery. This suggests the impact of technological changes on localization of seizure focus and/or developments in surgical procedures over time may not be a major contributor to seizure freedom at any period during the scope of our study. Others have also noted little difference between earlier and later surgical cases, 5 , 14 although a meta‐analysis found earlier surgery was associated with lower reported seizure freedom. 1 However, an important variable that has not been included in any analyses is the extent of difficulty experienced during preoperative seizure localization. Surgical cases have become more complex as the increasing sophistication of technology and preoperative investigations has facilitated surgery for individuals where localization is not straightforward. This characteristic may have confounded comparisons of outcome over time.
It is possible that some events classified as late seizures were nonepileptic events. 46 This is particularly relevant at extended postoperative periods, when follow‐up is conducted with patients who may not be receiving regular face‐to‐face medical consultations. An additional consideration in this study is that seizure recurrence has been defined as a single disabling seizure. The recurrence of any disabling seizure activity is important in terms of the impact on lifestyle and activities as well as understanding biological seizure mechanisms. However, late recurrences frequently respond to an adjustment of ASM, with few or no subsequent seizures. 2 , 44 , 47
As follow‐up begins to produce data relevant to decades of postoperative outcomes, the implications of this much wider timescale can be considered in future research. This requires considerable resources but has the capacity to offer possible insights into patient outcomes, epilepsy mechanisms, and seizure recurrence. Causes of late recurrence, and the reasons why high‐risk patients remain seizure‐free for extended periods, are two distinct research questions that it may be possible to address in more detail as follow‐up extends to lengthy time periods.
AUTHOR CONTRIBUTIONS
Anne M. McIntosh designed the study, collected data, undertook and interpreted analyses, and drafted the manuscript. Alex W. Wynd conducted patient follow‐up, collected data, and reviewed the manuscript. Samuel F. Berkovic supervised patient follow‐up, contributed to methodology, and reviewed manuscript drafts.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
SFB reports grants from NHMRC during the conduct of the study, and educational grants from UCB Pharma, Eisai, and SciGen. Neither of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
APPENDIX S1 Supporting information
ACKNOWLEDGMENTS
The authors thank Associate Professor Piero Perucca for review and feedback on the final draft of this paper. We also thank Clare Averill, who undertook postoperative patient follow‐up, and Associate Professor Gavin Fabinyi, who performed the surgery for most of the patients in these ATL and ET cohorts. In addition, we thank the neurologists who provided information that assisted with follow‐up. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
McIntosh AM, Wynd AW, Berkovic SF. Extended follow‐up after anterior temporal lobectomy demonstrates seizure recurrence 20+ years postsurgery. Epilepsia. 2023;64:92–102. 10.1111/epi.17440
REFERENCES
- 1. Tellez‐Zenteno JF, Dhar R, Wiebe S. Long‐term seizure outcomes following epilepsy surgery: a systematic review and meta‐analysis. Brain. 2005;128:1188–98. [DOI] [PubMed] [Google Scholar]
- 2. Petrik S, San Antonio‐Arce V, Steinhoff BJ, Syrbe S, Bast T, Scheiwe C, et al. Epilepsy surgery: late seizure recurrence after initial complete seizure freedom. Epilepsia. 2021;62(5):1092–104. [DOI] [PubMed] [Google Scholar]
- 3. McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long‐term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127(Pt 9):2018–30. [DOI] [PubMed] [Google Scholar]
- 4. Elsharkawy AE, Alabbasi AH, Pannek H, Oppel F, Schulz R, Hoppe M, et al. Long‐term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg. 2009;110(6):1135–46. [DOI] [PubMed] [Google Scholar]
- 5. Mohan M, Keller S, Nicolson A, Biswas S, Smith D, Osman Farah J, et al. The long‐term outcomes of epilepsy surgery. PLoS One. 2018;13(5):e0196274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, et al. The long‐term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378(9800):1388–95. [DOI] [PubMed] [Google Scholar]
- 7. Coleman H, McIntosh A, Wilson SJ. Identifying the trajectory of social milestones 15‐20 years after epilepsy surgery: realistic timelines for postsurgical expectations. Epilepsia Open. 2019;4(3):369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McIntosh AM, Berkovic SF. What happens now? Ongoing outcome after post‐temporal lobectomy seizure recurrence. Neurology. 2006;67(9):1671–3. [DOI] [PubMed] [Google Scholar]
- 9. McIntosh AM, Kalnins RM, Mitchell LA, Berkovic SF. Early seizures after temporal lobectomy predict subsequent seizure recurrence. Ann Neurol. 2005;57(2):283–8. [DOI] [PubMed] [Google Scholar]
- 10. McIntosh AM, Averill CA, Kalnins RM, Mitchell LA, Fabinyi GC, Jackson GD, et al. Long‐term seizure outcome and risk factors for recurrence after extratemporal epilepsy surgery. Epilepsia. 2012;53:970–8. [DOI] [PubMed] [Google Scholar]
- 11. Berkovic SF, McIntosh AM, Kalnins RM, Jackson GD, Fabinyi GC, Brazenor GA, et al. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology. 1995;45(7):1358–63. [DOI] [PubMed] [Google Scholar]
- 12. Sperling MR, Nei M, Zangaladze A, Sharan AD, Mintzer SE, Skidmore C, et al. Prognosis after late relapse following epilepsy surgery. Epilepsy Res. 2008;78(1):77–81. [DOI] [PubMed] [Google Scholar]
- 13. Ramesha KN, Mooney T, Sarma PS, Radhakrishnan K. Long‐term seizure outcome and its predictors in patients with recurrent seizures during the first year after temporal lobe resective epilepsy surgery. Epilepsia. 2011;52(5):917–24. [DOI] [PubMed] [Google Scholar]
- 14. Kelley K, Theodore WH. Prognosis 30 years after temporal lobectomy. Neurology. 2005;64(11):1974–6. [DOI] [PubMed] [Google Scholar]
- 15. Yoon HH, Kwon HL, Mattson RH, Spencer DD, Spencer SS. Long‐term seizure outcome in patients initially seizure‐free after resective epilepsy surgery. Neurology. 2003;61:445–50. [DOI] [PubMed] [Google Scholar]
- 16. Kelemen A, Barsi P, Eross L, Vajda J, Czirjak S, Borbely C, et al. Long‐term outcome after temporal lobe surgery—prediction of late worsening of seizure control. Seizure. 2006;15(1):49–55. [DOI] [PubMed] [Google Scholar]
- 17. Foldvary N, Nashold B, Mascha E, Thompson EA, Lee N, McNamara JO, et al. Seizure outcome after temporal lobectomy for temporal lobe epilepsy. A Kaplan‐Meier survival analysis. Neurology. 2000;54:630–4. [DOI] [PubMed] [Google Scholar]
- 18. Rasmussen TB. Surgical treatment of complex partial seizures: results, lessons, and problems. Epilepsia. 1983;24(Suppl 1):S65–76. [DOI] [PubMed] [Google Scholar]
- 19. Lamberink HJ, Otte WM, Blumcke I, Braun KPJ, European Epilepsy Brain Bank writing and study groups , European Reference Network EpiCARE . Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. 2020;19(9):748–57. [DOI] [PubMed] [Google Scholar]
- 20. Barba C, Rheims S, Minotti L, Grisotto L, Chabardes S, Guenot M, et al. Surgical outcome of temporal plus epilepsy is improved by multilobar resection. Epilepsia. 2022;63(4):769–76. [DOI] [PubMed] [Google Scholar]
- 21. Paillas JE, Gastaut H, Sedan R, Bureau M. Long‐term results of conventional surgical treatment for epilepsy. Delayed recurrence after a period of 10 years. Surg Neurol. 1983;20(3):189–93. [DOI] [PubMed] [Google Scholar]
- 22. Hemb M, Palmini A, Paglioli E, Paglioli EB, Costa da Costa J, Azambuja N, et al. An 18‐year follow‐up of seizure outcome after surgery for temporal lobe epilepsy and hippocampal sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(7):800–5. [DOI] [PubMed] [Google Scholar]
- 23. Rougier A, Dartigues JF, Commenges D, Claverie B, Loiseau P, Cohadon F. A longitudinal assessment of seizure outcome and overall benefit from 100 cortectomies for epilepsy. J Neurol Neurosurg Psychiatry. 1992;55(9):762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wieser HG, Ortega M, Friedman A, Yonekawa Y. Long‐term seizure outcomes following amygdalohippocampectomy. J Neurosurg. 2003;98(4):751–63. [DOI] [PubMed] [Google Scholar]
- 25. Radhakrishnan A, Abraham M, Vilanilam G, Menon R, Menon D, Kumar H, et al. Surgery for "long‐term epilepsy associated tumors (LEATs)": seizure outcome and its predictors. Clin Neurol Neurosurg. 2016;141:98–105. [DOI] [PubMed] [Google Scholar]
- 26. Luyken C, Blumcke I, Fimmers R, Urbach H, Elger CE, Wiestler OD, et al. The spectrum of long‐term epilepsy‐associated tumors: long‐term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003;44(6):822–30. [DOI] [PubMed] [Google Scholar]
- 27. Fong JS, Jehi L, Najm I, Prayson RA, Busch R, Bingaman W. Seizure outcome and its predictors after temporal lobe epilepsy surgery in patients with normal MRI. Epilepsia. 2011;52(8):1393–401. [DOI] [PubMed] [Google Scholar]
- 28. Paglioli E, Palmini A, Paglioli E, da Costa JC, Portuguez M, Martinez JV, et al. Survival analysis of the surgical outcome of temporal lobe epilepsy due to hippocampal sclerosis. Epilepsia. 2004;45(11):1383–91. [DOI] [PubMed] [Google Scholar]
- 29. Dupont S, Tanguy ML, Clemenceau S, Adam C, Hazemann P, Baulac M. Long‐term prognosis and psychosocial outcomes after surgery for MTLE. Epilepsia. 2006;47(12):2115–24. [DOI] [PubMed] [Google Scholar]
- 30. Fauser S, Essang C, Altenmuller DM, Staack AM, Steinhoff BJ, Strobl K, et al. Long‐term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia. 2015;56(1):66–76. [DOI] [PubMed] [Google Scholar]
- 31. Barba C, Rheims S, Minotti L, Guenot M, Hoffmann D, Chabardes S, et al. Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain. 2016;139(Pt 2):444–51. [DOI] [PubMed] [Google Scholar]
- 32. Schwartz TH, Jeha L, Tanner A, Bingaman W, Sperling MR. Late seizures in patients initially seizure free after epilepsy surgery. Epilepsia. 2006;47(3):567–73. [DOI] [PubMed] [Google Scholar]
- 33. Bujarski KA, Hirashima F, Roberts DW, Jobst BC, Gilbert KL, Roth RM, et al. Long‐term seizure, cognitive, and psychiatric outcome following trans‐middle temporal gyrus amygdalohippocampectomy and standard temporal lobectomy. J Neurosurg. 2013;119(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sala‐Padro J, Miro J, Rodriguez‐Fornells A, Rifa‐Ros X, Plans G, Santurino M, et al. Mapping connectivity fingerprints for presurgical evaluation of temporal lobe epilepsy. BMC Neurol. 2021;21(1):442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morgan VL, Sainburg LE, Johnson GW, Janson A, Levine KK, Rogers BP, et al. Presurgical temporal lobe epilepsy connectome fingerprint for seizure outcome prediction. Brain Commun. 2022;4(3):fcac128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He X, Doucet GE, Pustina D, Sperling MR, Sharan AD, Tracy JI. Presurgical thalamic "hubness" predicts surgical outcome in temporal lobe epilepsy. Neurology. 2017;88(24):2285–93. [DOI] [PubMed] [Google Scholar]
- 37. Lariviere S, Bernasconi A, Bernasconi N, Bernhardt BC. Connectome biomarkers of drug‐resistant epilepsy. Epilepsia. 2021;62(1):6–24. [DOI] [PubMed] [Google Scholar]
- 38. Pitkanen A, Ekolle Ndode‐Ekane X, Lapinlampi N, Puhakka N. Epilepsy biomarkers—toward etiology and pathology specificity. Neurobiol Dis. 2019;123:42–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moloney PB, Dugan P, Widdess‐Walsh P, Devinsky O, Delanty N. Genomics in the presurgical epilepsy evaluation. Epilepsy Res. 2022;184:106951. [DOI] [PubMed] [Google Scholar]
- 40. Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935‐1984. Epilepsia. 1993;34(3):453–68. [DOI] [PubMed] [Google Scholar]
- 41. Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Boston, MA: Little Brown; 1954. [Google Scholar]
- 42. Percy J, Zaveri H, Duckrow RB, Gerrard J, Farooque P, Hirsch LJ, et al. Beyond implantation effect? Long‐term seizure reduction and freedom following intracranial monitoring without additional surgical interventions. Epilepsy Behav. 2020;111:107231. [DOI] [PubMed] [Google Scholar]
- 43. Schmidt D, Baumgartner C, Loscher W. Seizure recurrence after planned discontinuation of antiepileptic drugs in seizure‐free patients after epilepsy surgery: a review of current clinical experience. Epilepsia. 2004;45(2):179–86. [DOI] [PubMed] [Google Scholar]
- 44. Schiller Y, Cascino GD, So EL, Marsh WR. Discontinuation of antiepileptic drugs after successful epilepsy surgery. Neurology. 2000;54(2):346–9. [DOI] [PubMed] [Google Scholar]
- 45. Jette N, Sander JW, Keezer MR. Surgical treatment for epilepsy: the potential gap between evidence and practice. Lancet Neurol. 2016;15(9):982–94. [DOI] [PubMed] [Google Scholar]
- 46. Markoula S, de Tisi J, Foong J, Duncan JS. De novo psychogenic nonepileptic attacks after adult epilepsy surgery: an underestimated entity. Epilepsia. 2013;54(12):e159–62. [DOI] [PubMed] [Google Scholar]
- 47. Rathore C, Jeyaraj MK, Dash GK, Wattamwar P, Baheti N, Sarma SP, et al. Outcome after seizure recurrence on antiepileptic drug withdrawal following temporal lobectomy. Neurology. 2018;91(3):e208–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Supporting information


