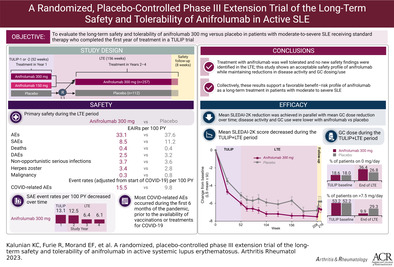
Abstract
Objective
To explore long‐term safety and tolerability of anifrolumab 300 mg compared with placebo in patients with systemic lupus erythematosus (SLE) who completed a Treatment of Uncontrolled Lupus via the Interferon Pathway (TULIP) trial and enrolled in the placebo‐controlled 3‐year long‐term extension (LTE) study (ClinicalTrials.gov identifier: NCT02794285).
Methods
In the blinded LTE study, patients continued anifrolumab 300 mg, switched from anifrolumab 150 mg to 300 mg, or were re‐randomized from placebo to receive either anifrolumab 300 mg or to continue placebo, administered every 4 weeks. Primary comparisons in the LTE study were between patients who received anifrolumab 300 mg or placebo throughout the TULIP and LTE studies. For rare safety events, comparisons included patients who received any anifrolumab dose during TULIP or LTE. When exposure differed, exposure‐adjusted incidence rates (EAIRs) per 100 patient‐years were calculated.
Results
In the LTE study, EAIRs of serious adverse events (SAEs) were 8.5 with anifrolumab compared with 11.2 with placebo; likewise, EAIRs of AEs leading to treatment discontinuation were 2.5 versus 3.2, respectively. EAIRs of non‐opportunistic serious infections were comparable between groups (3.7 with anifrolumab versus 3.6 with placebo). Exposure‐adjusted event rates of COVID‐related AEs, including asymptomatic infections, were 15.5 with anifrolumab compared with 9.8 with placebo. No COVID‐related AEs occurred in fully vaccinated individuals. EAIRs of malignancy and major acute cardiovascular events were low and comparable between groups. Anifrolumab was associated with lower cumulative glucocorticoid use and greater mean improvement in the SLE Disease Activity Index 2000, compared with placebo.
Conclusion
This LTE study represents the longest placebo‐controlled clinical trial performed in SLE to date. No new safety findings were identified in the LTE study, supporting the favorable benefit–risk profile of anifrolumab for patients with moderate‐to‐severe SLE receiving standard therapy.

INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by multisystem involvement ranging from mild to life‐threatening disease (1). Given the need for long‐term treatment, it is important to assess safety and efficacy of new therapies for SLE over an extended period. However, long‐term open‐label studies of biologic agents have left some questions remaining because of the lack of an appropriate control comparison group to distinguish the effects of the investigational treatment (2, 3).
Anifrolumab is a fully human IgG1 κ monoclonal antibody to the type I interferon (IFN) receptor that inhibits signaling of all type I IFNs (4). The phase III, randomized, double‐blind, placebo‐controlled Treatment of Uncontrolled Lupus via the Interferon Pathway 1 (TULIP‐1) and TULIP‐2 trials showed a positive benefit–risk profile for anifrolumab across a range of clinically important global and organ‐specific measures of disease activity in patients with active moderate‐to‐severe SLE despite standard therapy (5, 6), leading to its approval in multiple countries since 2021 (7, 8, 9, 10, 11). The 1‐year phase II MUSE trial, which was the first study to demonstrate anifrolumab efficacy in SLE patients (12), was followed by a 3‐year open‐label extension period. In the MUSE extension study, the safety profile, in particular serious adverse event and serious infection rates, was generally consistent with those observed in the initial MUSE 1‐year study, suggesting an acceptable long‐term safety profile with anifrolumab (13).
Reported here are results of the first placebo‐controlled long‐term trial in SLE. This study characterizes safety and tolerability of intravenous anifrolumab versus placebo in patients who had moderate‐to‐severe SLE despite standard therapy, and builds on prior evidence of long‐term safety and tolerability (5, 12, 13, 14) of the recommended 300 mg dose for anifrolumab administered every 4 weeks (7, 8, 9, 10, 11). To be eligible for this placebo‐controlled long‐term extension (LTE) study, patients had to complete a phase III TULIP trial through the 52‐week double‐blind treatment period (5, 6). Given the COVID‐19 pandemic emerging in the final year of this global multicenter extension study, this is also the first report on safety of an investigational biologic therapy in SLE during pre‐ and postvaccination periods of the COVID‐19 pandemic.
PATIENTS AND METHODS
Study design
This 3‐year, phase III, randomized, double‐blind, placebo‐controlled LTE study (ClinicalTrials.gov identifier: NCT02794285) (15) was conducted across 176 study sites in 24 countries in patients who completed the 52‐week double‐blind treatment period of 1 of the phase III TULIP trials (ClinicalTrials.gov identifiers: NCT02446912 for TULIP‐1 and NCT02446899 for TULIP‐2) (5, 6). Patients were required to have moderate‐to‐severe SLE at the time of TULIP‐1 or TULIP‐2 randomization and were allowed to participate in the extension study upon re‐consent; the start of the LTE was the end of the double‐blind treatment period of the TULIP trials. At the extension study enrollment, patients previously treated with anifrolumab 300 mg continued receiving anifrolumab 300 mg; patients previously treated with anifrolumab 150 mg in TULIP‐1 were switched to anifrolumab 300 mg; and patients previously randomized to receive placebo were re‐randomized 1:1 to receive anifrolumab 300 mg or placebo by an Interactive Voice/Web Response System algorithm. All patients were blinded with regard to treatment, to give an approximate final anifrolumab 300 mg–to‐placebo ratio of 4:1.
For primary safety, the main comparison during the 3‐year LTE study was between patients who received anifrolumab 300 mg in both TULIP and LTE trials (“LTE anifrolumab 300 mg”) and those who received placebo in both (“LTE placebo”). For rare safety events (e.g., malignancy, major acute cardiovascular events), data throughout the 4‐year TULIP and LTE (TULIP + LTE) period were used, and the main comparison groups were patients with any anifrolumab exposure, irrespective of dose (“all anifrolumab”) versus patients with any placebo exposure (“all placebo”). Data from patients randomized to receive placebo in TULIP and then anifrolumab 300 mg in LTE were included in the “all placebo” group before the first dose of anifrolumab in the LTE and in the “all anifrolumab” group afterwards. The safety profile of the 2 switch groups in the 3‐year LTE period (“anifrolumab 150 mg to 300 mg” and “placebo to anifrolumab 300 mg”) will also be described.
For efficacy assessments, including disease activity and steroid use, the main comparison groups during the 4‐year TULIP + LTE period were patients who received anifrolumab 300 mg or placebo in the TULIP studies and continued the same treatments throughout the LTE study (“combined anifrolumab 300 mg” versus “combined placebo” groups, respectively). Notably, the key difference between these groups and the LTE anifrolumab 300 mg and LTE placebo groups was timeframe analyzed (years 1–4 for TULIP + LTE versus years 2–4 for LTE‐only).
Patients received intravenous anifrolumab 300 mg or placebo every 4 weeks after LTE start (following the last visit in TULIP) for a total of up to 39 doses. After the 156‐week treatment period in the LTE study (last dose of investigational product given at week 204, the 152nd week of the LTE), patients continued for another 8‐week safety follow‐up period. This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice. All patients provided informed consent, and the study was approved by the ethics committee or institutional review board.
Patients
Full details of inclusion and exclusion criteria for the TULIP trials have been previously described (5, 6). For inclusion in the LTE study, patients had to complete the 52‐week treatment period, receiving anifrolumab or placebo in 1 of the TULIP trials. Patients were required to meet the following safety‐related criteria: repeat tuberculosis test showing no active tuberculosis (or if newly positive for latent tuberculosis, prophylaxis treatment was required), negative HIV test, and repeated Pap smear test without malignancy. Patients were excluded if they had any condition that would have interfered with study evaluation or if they were concurrently enrolled in another clinical study. Patients were also excluded if they received any above‐standard clinical doses of azathioprine; mycophenolate mofetil; mycophenolic acid; oral, subcutaneous, or intramuscular methotrexate; or mizoribine within the preceding 60 days.
The extension study was designed to reflect real‐world clinical practice, allowing investigators to add or change background standard care treatment, including immunosuppressants and glucocorticoids, based on clinical judgment. However, use of cyclophosphamide, other biologic drugs, intravenous immunoglobulin, or intravenous glucocorticoids was not permitted.
Study outcomes
The primary outcome was long‐term safety and tolerability as assessed by rates of adverse events (AEs), serious AEs (SAEs) including those leading to deaths, AEs leading to treatment discontinuation (DAEs), and AEs of special interest (AESIs). Safety information was collected at every visit. Exploratory efficacy outcomes included SLE Disease Activity Index 2000 (SLEDAI‐2K), Physician Global Assessment (PhGA), glucocorticoid use, flare incidence and severity, and the Systemic Lupus International Collaborating Clinics/American College Rheumatology Damage Index (SDI) global score. Further information on safety and efficacy assessments is described in Supplementary Methods (available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42392).
Exposure during the COVID‐19 pandemic
The LTE study started on June 30, 2016 (15) and continued during the COVID‐19 pandemic, which was declared on March 11, 2020 by the World Health Organization (16). Total exposure during the pandemic was calculated for each patient as:
Patients were considered to be fully vaccinated against COVID‐19 if they received an approved vaccine for the required number of doses, in line with standard COVID‐19 vaccination schedules in the relevant time period.
Statistical analysis
Baseline demographics and characteristics, all safety data (AEs, SAEs, deaths, DAEs, and AESIs), and most efficacy data (SLEDAI‐2K, PhGA, flare rates, and SDI) were summarized by descriptive statistics. Safety data were calculated as exposure‐adjusted incidence rates (EAIRs). COVID‐related safety events were described by event rates based on time at risk during the pandemic. Mean change from baseline in SLEDAI‐2K was determined using an analysis of covariance, including baseline value (continuous), treatment group, visit, and randomization stratification factors (type I IFN gene signature test result at screening and glucocorticoid dose at baseline). For glucocorticoid use, standardized area under the curve (AUC) and proportion of patients by glucocorticoid dose were both presented by treatment group and by year with summary statistics. Additional details on exposure and sensitivity analyses for glucocorticoid use and assessment of flares are provided in the Supplementary Methods (https://onlinelibrary.wiley.com/doi/10.1002/art.42392).
RESULTS
Treatment randomization, patient disposition, demographics, and baseline characteristics
Of 639 patients who completed treatment in the TULIP studies, 547 enrolled in the LTE study and were randomized to receive ≥1 dose of treatment (Figure 1 and Supplementary Figure 1, https://onlinelibrary.wiley.com/doi/10.1002/art.42392). There were 257 patients who continued anifrolumab 300 mg (LTE anifrolumab 300 mg) and 67 patients transitioned from anifrolumab 150 mg to 300 mg. The 223 patients who received placebo in TULIP‐1 or TULIP‐2 were re‐randomized 1:1 to receive anifrolumab 300 mg (n = 111) or placebo (n = 112; LTE placebo) in the LTE.
Figure 1.
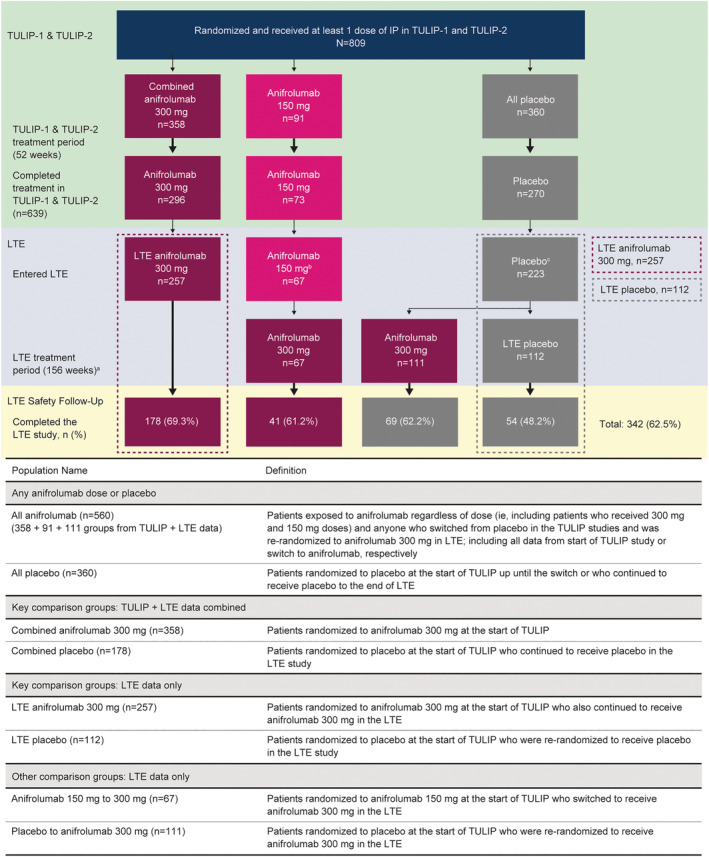
Patients included in the TULIP‐1 or TULIP‐2 trials and LTE study: treatment randomization and treatment group definitions. aThere was an 8‐week safety follow‐up period. bPatients randomized to receive anifrolumab 150 mg were all in TULIP‐1. cPatients were re‐randomized to receive anifrolumab 300 mg or placebo for the LTE study. TULIP‐1 = Treatment of Uncontrolled Lupus via the Interferon Pathway 1; IP = investigational product; LTE = long‐term extension.
Among patients who would go on to comprise primary LTE safety comparison groups (LTE anifrolumab 300 mg and LTE placebo groups), demographics and baseline disease characteristics at the start of TULIP studies were generally well balanced between groups (Table 1), and similar proportions of patients were receiving glucocorticoids or immunosuppressants. Baseline characteristics at the first LTE visit are also included in Table 1. Use of concomitant medications, other than glucocorticoids, remained broadly comparable during the LTE study between the LTE anifrolumab 300 mg and LTE placebo groups; further information on concomitant medications and vaccinations are summarized in the Supplementary Results and Supplementary Table 1 (https://onlinelibrary.wiley.com/doi/10.1002/art.42392). For patients who continued to the LTE, the mean ± SD SLEDAI‐2K global scores at baseline in the TULIP trials were 11.2 ± 3.7 in the LTE anifrolumab 300 mg group and 11.3 ± 3.6 in the LTE placebo group. These were consistent with the overall TULIP population (5, 6).
Table 1.
Demographics and SLE disease characteristics at baseline in the TULIP trials and at LTE study entry (week 52) for patients who continued treatment in the LTE study*
| TULIP baseline (week 0) | LTE entry (week 52) | |||
|---|---|---|---|---|
| Characteristic | LTE anifrolumab 300 mg (n = 257) | LTE placebo (n = 112) | LTE anifrolumab 300 mg (n = 257) | LTE placebo (n = 112) |
| Age, mean ± SD years | 43.4 ± 12.0 | 41.4 ± 11.5 | – | – |
| Female sex | 237 (92.2) | 103 (92.0) | – | – |
| Race | – | – | ||
| White | 173 (67.3) | 77 (68.8) | – | – |
| Black | 28 (10.9) | 11 (9.8) | – | – |
| Asian | 33 (12.8) | 10 (8.9) | – | – |
| Other | 15 (5.8) | 11 (9.8) | – | – |
| Hispanic or Latino | 54 (21.0) | 28 (25.0) | – | – |
| Geographic region | – | – | ||
| US/Canada | 98 (38.1) | 44 (39.3) | ||
| Europe | 90 (35.0) | 41 (36.6) | – | – |
| Latin America | 33 (12.8) | 15 (13.4) | – | – |
| Asia Pacific | 31 (12.1) | 8 (7.1) | – | – |
| Other (rest of world) | 5 (1.9) | 4 (3.6) | – | – |
| Time from initial SLE diagnosis to randomization, median (range) months | 92.0 (0–555) | 80.5 (6–503) | – | – |
| SLEDAI‐2K | ||||
| Mean ± SD | 11.2 ± 3.7 | 11.3 ± 3.6 | 4.9 ± 3.5 | 5.9 ± 4.3 |
| Score ≥10 | 184 (71.6) | 80 (71.4) | 30 (11.7) | 25 (22.3) |
| PhGA score, mean ± SD | 1.8 ± 0.4 | 1.8 ± 0.4 | 0.7 ± 0.5 | 0.9 ± 0.6 |
| SDI global score, mean ± SD | 0.6 ± 1.1 | 0.6 ± 0.9 | 0.7 ± 1.1 | 0.7 ± 0.9 |
| SDI global score ≥1 | 90 (35.0) | 46 (41.1) | 97 (37.7) | 51 (45.5) |
| Type I IFN gene signature high | 206 (80.2) | 93 (83.0) | – | – |
| ANA positive | 229 (89.1) | 99 (88.4) | 211 (82.1) | 97 (86.6) |
| Anti‐dsDNA positive | 113 (44.0) | 38 (33.9) | 93 (36.2) | 38 (33.9) |
| Abnormal (low) complement C3 | 90 (35.0) | 36 (32.1) | 77 (30.0) | 38 (33.9) |
| Abnormal (low) complement C4 | 56 (21.8) | 19 (17.0) | 37 (14.4) | 22 (19.6) |
| Baseline SLE treatments | ||||
| GCs (prednisone or equivalent) | 208 (80.9) | 92 (82.1) | 184 (71.6) | 87 (77.7) |
| Antimalarials | 171 (66.5) | 83 (74.1) | 169 (65.8) | 81 (72.3) |
| Azathioprine | 42 (16.3) | 18 (16.1) | 41 (16.0) | 17 (15.2) |
| Methotrexate | 45 (17.5) | 26 (23.2) | 44 (17.1) | 26 (23.2) |
| Mycophenolate | 37 (14.4) | 14 (12.5) | 36 (14.0) | 13 (11.6) |
| NSAIDs | 27 (10.5) | 14 (12.5) | 28 (10.9) | 14 (12.5) |
Except where indicated otherwise, values are the number (%). SLE = systemic lupus erythematosus; TULIP = Treatment of Uncontrolled Lupus via the Interferon Pathway; LTE = long‐term extension; SLEDAI‐2K = SLE Disease Activity Index 2000; PhGA = Physician Global Assessment; SDI = Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; IFN = interferon; ANA = antinuclear antibody; anti‐dsDNA = anti–double‐stranded DNA; GCs = glucocorticoids; NSAIDs = nonsteroidal antiinflammatory drugs.
Of patients in the LTE anifrolumab 300 mg group, 69.3% (178 of 257) completed the 3‐year extension study, compared with 48.2% (54 of 112) of patients in the LTE placebo group (Supplementary Figure 1, https://onlinelibrary.wiley.com/doi/10.1002/art.42392). The most commonly reported reasons for discontinuation were withdrawal by patient and lack of efficacy. Eight patients discontinued treatment because of the COVID‐19 pandemic (LTE anifrolumab 300 mg group, n = 7 [2.7%]; LTE placebo group, n = 1 [0.9%]). During the extension study, overall anifrolumab exposure in the LTE anifrolumab 300 mg group was 683.5 patient‐years compared with 250.3 patient‐years of exposure to placebo in the LTE placebo group. The total exposure to anifrolumab across all doses and all groups in the TULIP studies and the 3‐year LTE study was 1,568 patient‐years.
Safety
The safety profile of anifrolumab in SLE during the TULIP studies has been published (5, 6). Safety results for the LTE study alone (years 2–4) are shown in Table 2, and safety results for the TULIP + LTE studies (4 years from the start of TULIP to the end of the LTE study) are shown in Table 3.
Table 2.
AEs, SAEs, deaths, AESIs, and EAIRs in any category during treatment and follow‐up during weeks 52–216 (LTE years 2–4)*
| LTE anifrolumab 300 mg (n = 257; exposure 683.5 patient‐years†) | LTE placebo (n = 112; exposure 250.3 patient‐years†) | |||
|---|---|---|---|---|
| No. (%) | EAIR (per 100 patient‐years)‡ | No. (%) | EAIR (per 100 patient‐years)‡ | |
| Any AE | 226 (87.9) | 33.1 | 94 (83.9) | 37.6 |
| Any SAE (including events with outcome of death) | 58 (22.6) | 8.5 | 28 (25.0) | 11.2 |
| Any AE with outcome of death | 3 (1.2) | 0.4 | 1 (0.9) | 0.4 |
| Any DAE | 17 (6.6) | 2.5 | 8 (7.1) | 3.2 |
| Any AE of severe intensity | 43 (16.7) | 6.3 | 13 (11.6) | 5.2 |
| Any AESI | 75 (29.2) | 11.0 | 24 (21.4) | 9.6 |
| Any AESI of non‐opportunistic serious infections | 25 (9.7) | 3.7 | 9 (8.0) | 3.6 |
| Any AESI of herpes zoster | 23 (8.9) | 3.4 | 7 (6.3) | 2.8 |
| Any AESI of latent tuberculosis§ | 16 (6.2) | 2.3 | 2 (1.8) | 0.8 |
| Any AESI of influenza | 15 (5.8) | 2.2 | 2 (1.8) | 0.8 |
| Any AESI of major acute cardiovascular events¶ | 5 (1.9) | 0.7 | 3 (2.7) | 1.2 |
| Any AESI of malignancy | 2 (0.8) | 0.3 | 2 (1.8) | 0.8 |
| Any AESI of anaphylaxis | 0 | 0 | 0 | 0 |
| Any AESI of opportunistic infections | 0 | 0 | 3 (2.7) | 1.2 |
| Any AESI of vasculitis | 0 | 0 | 0 | 0 |
Data presented are solely from the extension period. Only analyses of descriptive statistics were performed. AE = adverse event; SAE = serious AE; DAE = AE leading to treatment discontinuation; AESI = AE of special interest; LTE = long‐term extension.
Exposure in days for each patient was calculated as: the earlier of either (i.e., date of last dose of treatment + 84 days, or date of study discontinuation) − date of first dose of treatment + 1 day.
The exposure‐adjusted incident rate (EAIR) per 100 patient‐years was defined as the number of patients with the specific event divided by the total exposure in years × 100. The exposure time was defined as from the date of first administration of treatment to death, end of treatment + 84 days, or end of study, whichever came first.
Latent tuberculosis was defined as a positive interferon‐γ release assay result. All patients were required to be tested at least annually and, in some cases, more often, depending on the result. No active cases of tuberculosis were reported.
According to the cardiovascular event adjudication committee.
Table 3.
AEs, SAEs, deaths, AESIs, and EAIRs in any category during treatment and follow‐up during weeks 0–216 (TULIP + LTE years 1–4)*
| All anifrolumab (n = 560; exposure 1,568.0 patient‐years†) | Combined anifrolumab 300 mg (n = 358; exposure 1,026.2 patient‐years†) | All placebo (n = 360; exposure 587.1 patient‐years†) | ||||
|---|---|---|---|---|---|---|
| No. (%) | EAIR (per 100 patient‐years)‡ | No. (%) | EAIR (per 100 patient‐years)‡ | No. (%) | EAIR (per 100 patient‐years)‡ | |
| Any AE | 522 (93.2) | 33.3 | 338 (94.4) | 32.9 | 318 (88.3) | 54.2 |
| Any SAE (including events with outcome of death) | 147 (26.3) | 9.4 | 92 (25.7) | 9.0 | 91 (25.3) | 15.5 |
| Any AE with outcome of death | 10 (1.8) | 0.6 | 5 (1.4) | 0.5 | 2 (0.6) | 0.3 |
| Any DAE | 59 (10.5) | 3.8 | 35 (9.8) | 3.4 | 30 (8.3) | 5.1 |
| Any AE of severe intensity | 102 (18.2) | 6.5 | 67 (18.7) | 6.5 | 46 (12.8) | 7.8 |
| Any death of COVID‐19 infection | 2 (0.4) | 0.1 | 1 (0.3) | 0.1 | 0 | 0 |
| Any AESI | 180 (32.1) | 11.5 | 113 (31.6) | 11.0 | 61 (16.9) | 10.4 |
| Any AESI of herpes zoster | 75 (13.4) | 4.8 | 45 (12.6) | 4.4 | 13 (3.6) | 2.2 |
| Any AESI of non‐opportunistic serious infections | 55 (9.8) | 3.5 | 37 (10.3) | 3.6 | 29 (8.1) | 4.9 |
| Non‐opportunistic serious infections of COVID‐19 | 9 (1.6) | 0.6 | 6 (1.7) | 0.6 | 0 | 0 |
| Any AESI of influenza | 36 (6.4) | 2.3 | 20 (5.6) | 1.9 | 11 (3.1) | 1.9 |
| Any AESI of latent tuberculosis§ | 27 (4.8) | 1.7 | 20 (5.6) | 1.9 | 4 (1.1) | 0.7 |
| Any AESI of opportunistic infections | 3 (0.5) | 0.2 | 1 (0.3) | 0.1 | 4 (1.1) | 0.7 |
| Any AESI of anaphylaxis | 1 (0.2) | 0.1 | 0 | 0 | 0 | 0 |
| Any AESI of malignancy | 12 (2.1) | 0.8 | 7 (2.0) | 0.7 | 4 (1.1) | 0.7 |
| Any AESI of major acute cardiovascular events | 12 (2.1) | 0.8 | 6 (1.7) | 0.6 | 3 (0.8) | 0.5 |
| Any AESI of vasculitis | 0 | 0 | 0 | 0 | 0 | 0 |
Data presented are combined from the Treatment of Uncontrolled Lupus via the Interferon Pathway (TULIP) trials and the extension study. Only descriptive statistics were performed. See Table 2 for other definitions.
Exposure in days for each patient was calculated as: the earlier of either (i.e., date of last dose of treatment + 84 days, or date of study discontinuation) − date of first dose of treatment + 1 day.
The EAIR per 100 patient‐years was defined as the number of patients with the specific event divided by the total exposure in years ×100. The exposure time was defined as from the date of first administration of treatment to death, end of treatment + 84 days, or end of study, whichever came first.
Latent tuberculosis was defined as a positive interferon‐γ release assay result. All patients were required to be tested at least annually and, in some cases, more often, depending on the result. No active cases of tuberculosis were reported.
Focusing on a comparison of the LTE anifrolumab 300 mg group versus the LTE placebo group, EAIRs per 100 patient‐years of any AE (33.1 versus 37.6), any SAE including events with outcome of death (8.5 versus 11.2), and any DAE (2.5 versus 3.2) were all generally similar between groups (Table 2). Safety profiles of the 2 switch groups in the LTE study are included in Supplementary Table 2 (https://onlinelibrary.wiley.com/doi/10.1002/art.42392). The long‐term safety profile of anifrolumab in patients who received any dose (all anifrolumab group) was consistent with that observed in the combined TULIP + LTE data set (combined anifrolumab 300 mg group) (Table 3).
Safety during the LTE period
The most common AEs in the LTE by EAIRs per 100 patient‐years were nasopharyngitis (9.7 versus 5.5), urinary tract infection (8.5 versus 6.3), and upper respiratory tract infection (8.3 versus 7.2) in the LTE anifrolumab 300 mg and LTE placebo groups, respectively (Supplementary Table 3, https://onlinelibrary.wiley.com/doi/10.1002/art.42392). In the same groups, the most frequently reported types of SAEs were in the system organ class of infections and infestations (4.3 and 4.7 per 100 patient‐years, respectively).
Rates of any SAEs among patients treated with anifrolumab decreased over time and were lower during LTE than during the first year of treatment in TULIP trials (5, 6). The exposure‐adjusted event rates based on time at risk, and the corresponding number and percentage of patients is as follows for the all anifrolumab group: 13.1 for year 1 (n = 53 [11.8%]); 12.5 for year 2 (n = 49 [11.3%]); 6.4 for year 3 (n = 23 [5.9%]); 6.1 for year 4 (n = 19 [5.6%]). A similar pattern of decline in SAE rates was observed in the all placebo group: 22.0 for year 1 (n = 68 [18.9%]); 12.4 for year 2 (n = 12 [10.7%]); 10.6 for year 3 (n = 8 [8.9%]); 4.9 for year 4 (n = 3 [4.2%]).
Among patients in LTE anifrolumab 300 mg and LTE placebo groups, overall rates of any DAE were low (2.5 and 3.2, respectively), and no DAE was reported in >2 patients per group (Supplementary Table 4, https://onlinelibrary.wiley.com/doi/10.1002/art.42392). DAEs of COVID‐19, pneumonia, and postherpetic neuralgia were reported in 2 patients (0.8%), each in the LTE anifrolumab 300 mg group; DAEs of COVID‐19 and SLE were observed in 1 patient (0.9%) and 2 patients (1.8%), respectively, in the LTE placebo group. Rates of any DAEs among patients treated with anifrolumab 300 mg group decreased over the LTE period and were lower than during the first year of treatment in the TULIP trials (5, 6) (exposure‐adjusted event rates based on time at risk in the combined anifrolumab 300 mg group: 5.3 for year 1; 2.9 for year 2; 3.2 for year 3; 1.6 for year 4). Similarly, DAE rates decreased over time in those treated with placebo (all placebo group: 6.6 for year 1; 3.0 for year 2; 2.6 for year 3; 4.8 for year 4).
During the LTE, rates of AESIs were low in both LTE anifrolumab 300 mg and LTE placebo groups (Table 2). Rates per 100 patient‐years of non‐opportunistic serious infections (3.7 versus 3.6) were similar between groups, though rates of latent tuberculosis (2.3 versus 0.8) and influenza (2.2 versus 0.8) were numerically higher in the LTE anifrolumab 300 mg group than the LTE placebo group. Of note, latent tuberculosis was defined as a positive IFNγ release assay (17). There were no cases of active tuberculosis in either group, and no opportunistic infections were reported in the LTE anifrolumab 300 mg group.
Over the 3‐year LTE period, rates per 100 patient‐years of herpes zoster (HZ) were 3.4 in the LTE anifrolumab 300 mg group and 2.8 in the LTE placebo group (Table 2), 6.6 in the anifrolumab 150 mg to 300 mg group, and 4.9 in the placebo to anifrolumab 300 mg group (Supplementary Table 2, https://onlinelibrary.wiley.com/doi/10.1002/art.42392). Considering total anifrolumab exposure, rates of HZ by year decreased over time and were lower during the LTE period than during the first year of treatment in the TULIP trials (exposure‐adjusted event rates based on time at risk in the all anifrolumab group: 6.8 for year 1; 5.7 for year 2; 3.9 for year 3; 2.9 for year 4). A total of 48 patients had HZ during the LTE period with anifrolumab (LTE anifrolumab 300 mg group [n = 23]; placebo to anifrolumab 300 mg group [n = 14], anifrolumab 150 mg to 300 mg group [n = 11]). Compared with 3 DAEs during the 1 year of TULIP, there were 5 DAEs due to HZ in the 3‐year LTE study (year 2 [n = 4] and year 3 [n = 1]). There was 1 DAE in the LTE anifrolumab 300 mg group, 1 in the placebo to anifrolumab 300 mg group, and 3 in the anifrolumab 150 mg to 300 mg group.
Deaths during the LTE period alone and during the TULIP + LTE period
The EAIR of any AE with the outcome of death was 0.4 per 100 patient‐years in both the LTE anifrolumab 300 mg and LTE placebo groups, including 3 deaths resulting from infections (1 attributable to COVID‐19 and 2 to pneumonia) among patients who had been treated with anifrolumab, and 1 death from a major acute cardiovascular event (acute myocardial infarction) in a patient treated with placebo (Table 2). Two additional deaths due to COVID‐19 occurred during the LTE in the placebo to anifrolumab 300 mg group. All COVID‐related safety data are addressed in a separate section below. When considering all anifrolumab and placebo exposure across 4 years, there were a total of 12 deaths, including the 3 previously reported in TULIP trials (5, 6) and the 6 mentioned above that occurred during the LTE. Ten of these deaths were in the all anifrolumab group, and 2 deaths were reported in the all placebo group, yielding EAIRs of 0.6 and 0.3, respectively (Table 3).
Rare safety events during the TULIP + LTE period
To be comprehensive and include the greatest exposure, rare AEs were summarized over the 4‐year exposure period (all anifrolumab [n = 560], total exposure 1,568.0 patient‐years; all placebo [n = 360], total exposure 587.1 patient‐years). A numerically lower rate of opportunistic infections was observed in the all anifrolumab group compared with the all placebo group (0.2 versus 0.7) (Table 3). Comparably low rates of malignancy (0.8 versus 0.7), anaphylaxis (0.1 versus 0.0), and major acute cardiovascular events (0.8 versus 0.5) were observed in both groups. Only nonmelanoma skin cancers were reported in >1 patient: basal cell carcinoma and squamous cell carcinoma (n = 2 and n = 3, respectively).
COVID‐related AEs, SAEs, and deaths during the COVID‐19 pandemic
Although COVID‐19 was not a prespecified AESI, in view of the pandemic and SLE community interest in this topic, COVID‐19 safety data are addressed in this section. Among patients in the LTE at the start of the pandemic being treated with either anifrolumab 300 mg (n = 325) or placebo (n = 64), total treatment exposure was 227.7 and 42.7 patient‐years, respectively (Table 4). Concomitant use of immunosuppressant therapy was noted in 4 AE‐related deaths due to pneumonia (combined anifrolumab 300 mg [n = 1]), COVID‐19 (combined anifrolumab 300 mg [n = 1]; placebo to anifrolumab 300 mg [n = 1]), and unknown causes (placebo to anifrolumab 300 mg [n = 1]).
Table 4.
COVID‐19–related AEs and event rates during treatment and follow‐up of the LTE study*
| All anifrolumab (n = 325 at start of pandemic†; exposure during pandemic 227.7 patient‐years‡) | LTE anifrolumab 300 mg (n = 201 at start of pandemic†; exposure during pandemic 143.5 patient‐years‡) | LTE placebo (n = 64 at start of pandemic†; exposure during pandemic 42.7 patient‐years‡) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AE | SAE | AE with outcome of death | AE | SAE | AE with outcome of death | AE | SAE | AE with outcome of death | |
| Patients with an event, no. (%) | 33 (10.2) | 16 (4.9) | 3 (0.9) | 22 (10.9) | 10 (5.0) | 1 (0.5) | 4 (6.3) | 1 (1.6) | 0 |
| Time at risk, patient‐years§ | 213.6 | 221.6 | 227.4 | 134.1 | 140.4 | 143.4 | 40.7 | 42.4 | 42.7 |
| Event rate | 15.5 | 7.2 | 1.3 | 16.4 | 7.1 | 0.7 | 9.8 | 2.4 | 0.0 |
| (95% CI)¶ | (10.6–21.7) | (4.1–11.7) | (0.3–3.9) | (10.3–24.8) | (3.4–13.1) | (0.02–3.9) | (2.7–25.1) | (0.1–13.1) | (0.00–8.6) |
95% CI = 95% confidence interval (see Table 2 for other definitions).
The start date of the COVID‐19 pandemic was March 11, 2020, as declared by the World Health Organization.
Exposure during the pandemic for each patient was calculated as: the end of period − start date of the COVID‐19 pandemic + 1. End of period was the earliest of the following: date of last dose of treatment + 84 days, date of study discontinuation, death, or withdrawal of consent.
Total exposure for number (%) data. The time at risk was defined as time (including start and end date) from the start date of the COVID‐19 pandemic to the date of first event or end of period, whichever came first.
The event rate per 100 patient‐years was defined as the number of patients with an event divided by the total time at risk during the pandemic in years × 100. Time at risk was defined as the date of start of the pandemic to death, end of treatment + 84 days, or end of study, whichever came first. When reporting events that occurred during treatment only, end of period + 28 days instead of 84 days was considered.
Event rates (based on time at risk during the pandemic) for COVID‐related AEs were 15.5 in patients receiving all anifrolumab 300 mg compared with 9.8 in patients receiving placebo. Rates of COVID‐related SAEs were higher with anifrolumab 300 mg compared with placebo during the LTE study (7.2 versus 2.4) (Table 4). Of the 33 patients receiving anifrolumab 300 mg who reported a COVID‐related AE, 16 were serious (48.5%); 1 patient receiving placebo reported a COVID‐related SAE (1 of 4 [25%]) (Supplementary Figure 2, https://onlinelibrary.wiley.com/doi/10.1002/art.42392). Of note, asymptomatic positive COVID‐19 tests were considered COVID‐related AEs. No COVID‐related AEs occurred in patients after they were fully vaccinated. Twenty‐one patients in the anifrolumab 300 mg group and 9 in the placebo group were fully vaccinated against COVID‐19. The 3 deaths in patients receiving anifrolumab 300 mg each occurred in different countries within the first 6 months of the pandemic.
Immunogenicity
Treatment‐emergent antidrug antibodies were detected in 3.1% of patients (17 of 548) receiving all anifrolumab throughout the 4‐year treatment period, with no trend or pattern to suggest any association with AEs.
Efficacy
All efficacy data were evaluated in the combined anifrolumab 300 mg group (n = 358) and compared with the combined placebo group (n = 178) throughout the TULIP and LTE periods. For patients who continued into the LTE study, baseline (start of TULIP) mean ± SD SLEDAI‐2K scores were 11.4 ± 3.8 in the combined anifrolumab 300 mg group and 11.3 ± 3.9 in the combined placebo group. At LTE start (week 52), mean ± SD scores were 5.1 ± 3.5 and 6.0 ± 4.1, respectively. During the LTE study, patients in the combined anifrolumab 300 mg group had greater mean improvement in SLEDAI‐2K, with sustained improvement over time compared with the combined placebo group (Figure 2A); the mean SLEDAI‐2K score steadily decreased from baseline to week 52 in the TULIP period and continued to decrease during the LTE period to week 208 in the combined anifrolumab 300 mg group. Similar patterns were observed in the mean clinical SLEDAI‐2K score changes from baseline to week 208 (Supplementary Figure 3, https://onlinelibrary.wiley.com/doi/10.1002/art.42392). The mean PhGA score decreased from 1.8 at TULIP baseline to 0.6 at week 208 in the combined anifrolumab 300 mg group and from 1.8 to 0.7 in the combined placebo group (Supplementary Table 5, https://onlinelibrary.wiley.com/doi/10.1002/art.42392).
Figure 2.

Efficacy results during the TULIP trials and long‐term extension study (TULIP + LTE) period in the combined anifrolumab 300 mg group compared with the combined placebo group. A, Change in the mean Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI‐2K) score from baseline to week 216. B, Cumulative glucocorticoid (GC) dose standardized area under the curve (AUC) during the 4‐year study period. C, Percentage of patients by glucocorticoid dose group at each year during the TULIP trials and the LTE study (excluding 3 patients from the combined anifrolumab 300 mg group who received a glucocorticoid dose >40 mg/day at TULIP study baseline). The combined anifrolumab 300 mg group includes patients randomized to receive anifrolumab 300 mg at the start of a TULIP study and to continue receiving anifrolumab 300 mg in the LTE study. The combined placebo group includes patients randomized to receive placebo at the start of a TULIP study and to continue receiving placebo in the LTE study. LS = least‐squares.
For each of the 4 years during the TULIP + LTE period, the cumulative glucocorticoid dose was lower among patients in the combined anifrolumab 300 mg group compared with the combined placebo group (standardized AUC; Figure 2B), which excluded 3 patients in the anifrolumab group due to missing data. Reduction in SLEDAI‐2K was achieved in parallel with reduction of the mean glucocorticoid dose (Supplementary Figure 4, https://onlinelibrary.wiley.com/doi/10.1002/art.42392). The proportion of patients receiving mean glucocorticoid doses >7.5 mg/day was lower in the combined anifrolumab 300 mg group compared with the combined placebo group over the 4 years spanning the TULIP and extension periods (Figure 2C). At the end of LTE study (year 4), 9.9% of patients in the combined anifrolumab 300 mg group were receiving >7.5 mg/day of glucocorticoids compared with 29.3% in the combined placebo group.
The overall annualized flare rate was 0.1 in the combined anifrolumab 300 mg group and 0.2 in the combined placebo group; the majority of flares were mild to moderate in severity.* During the LTE study, the mean global SDI score remained stable in both groups (Supplementary Figure 5, https://onlinelibrary.wiley.com/doi/10.1002/art.42392). Changes from baseline in global SDI score and time to first SDI worsening are summarized in the Supplementary Results.
DISCUSSION
Anifrolumab is a first‐in‐class therapy recently approved for moderate‐to‐severe SLE despite standard care, and long‐term data reported here are important for prescribing physicians treating patients with this chronic disease (5, 6, 7, 8, 9, 10, 12, 18, 19, 20). In particular, these data described the first long‐term placebo‐controlled study in SLE and additionally captured a period of the global COVID‐19 pandemic. This study builds on existing evidence from the MUSE open‐label extension study (13) and shows that treatment with anifrolumab was well tolerated and had an acceptable long‐term safety profile while maintaining reductions in disease activity and glucocorticoid usage.
Incidence of SAEs in the first year of LTE (year 2) with anifrolumab treatment was similar to observations during the TULIP studies (5, 6), and rates decreased over time in the LTE study. The rate of DAEs over time remained largely consistent in the anifrolumab group and does not fully account for the observed decrease in SAE rates. Per convention, we report safety data as EAIRs to account for differences in exposure time and discontinuation between the LTE anifrolumab 300 mg and LTE placebo groups. Rates of non‐opportunistic serious infections were low and comparable with placebo in this LTE study.
Of particular interest, the increased rate of HZ reactivation reported in TULIP studies (5, 6) in the context of type I IFN blockade was further assessed here. The rate of HZ with anifrolumab 300 mg observed in this study was lower than that observed in pooled anifrolumab 300 mg results from TULIP‐1, TULIP‐2, and MUSE studies (LTE: 3.4; pooled phase II and phase III data: 6.9) (18). This suggests that risk of HZ with anifrolumab may be highest during the first year of treatment. Although 48 patients had HZ during the LTE period with anifrolumab treatment (LTE anifrolumab 300 mg [n = 23], placebo to anifrolumab 300 mg [n = 14], anifrolumab 150 mg to 300 mg [n = 11]), only 5 patients discontinued the LTE study due to HZ, suggesting that many patients continued therapy long‐term. Notably, only 1 of the DAE cases of HZ occurred in the LTE anifrolumab 300 mg group, and the remaining 4 cases with anifrolumab in the LTE were in the 2 switch groups. Considering that the Shingrix vaccine was only licensed for those <50 years of age toward the end of the LTE study (July 2021) (21) and thus very few patients (n = 5) received HZ vaccinations during the 4‐year TULIP + LTE period, vaccination status was likely not a major contributor to observed decreased HZ rates over time. Most cases in the LTE study were mild to moderate and responded to treatment with antiviral drugs. Moreover, overall HZ prevalence with anifrolumab in the LTE study (23 of 257 patients [8.9%] in the LTE anifrolumab 300 mg group) was similar to that reported with long‐term treatment with belimumab (63 of 735 patients [8.6%]) in an extension of the phase III BLISS‐52 and BLISS‐76 trials in SLE (22).
Latent tuberculosis in this study was defined as a positive IFNγ release assay, which can lead to indeterminate results in patients with active SLE due to the use of immunomodulatory therapy and/or common occurrence of lymphopenia (17). Although there were higher rates of latent tuberculosis reported in the anifrolumab group compared with placebo, perhaps due to improved disease control in the anifrolumab group, there were no cases of active tuberculosis. This is consistent with previous findings showing improvements in SLE‐associated lymphopenia with anifrolumab (23).
Malignancy rates in this study were low and comparable with placebo. Only non‐melanoma skin cancers (basal cell carcinoma and squamous cell carcinoma) were reported in >1 patient. Long‐term malignancy risk will be further evaluated in a planned post‐authorization safety study. Major cardiovascular events adjudicated by an independent cardiovascular adjudication committee were infrequent and comparable between anifrolumab and placebo. No patients experienced anaphylaxis in the LTE study.
To our knowledge, this is the only placebo‐controlled study in patients with SLE to report continuous data spanning the start as well as the pre‐ and postvaccination periods of the COVID‐19 pandemic. Studies have found that patients with SLE have a higher risk of severe COVID‐19 viral infection and poorer outcomes compared with the general population, and there have been concerns that some treatments for SLE could increase the risk of more severe viral disease (24). Some studies suggest that patients with SLE receiving immunosuppressant therapies and those with B cell depletion have a greater risk of contracting COVID‐19 and experiencing poor outcomes (25, 26, 27, 28). Type I IFN is elevated in most patients with SLE and may play a role in the disease course of COVID‐19 (27), although large randomized clinical trials of recombinant IFN in severe COVID‐19 failed to demonstrate any benefit (29). Further, type I IFN responses are important in host viral defense, and their absence (i.e., autoantibody states) has been associated with a high risk of severe COVID‐19 and death (30). Therefore, it was of particular interest to evaluate COVID‐related AEs in patients treated with the type I IFN inhibitor anifrolumab.
In the present study of long‐term anifrolumab treatment, rates of COVID‐related SAEs were higher in patients treated with anifrolumab compared with placebo, although population numbers were low, especially in the placebo group, by the time the pandemic started. Approximately 50% of COVID‐related AEs were serious in the all anifrolumab group, compared with 25% in the LTE placebo group. However, most of the COVID‐related SAEs and all 3 deaths occurred during the first 6 months of the pandemic, prior to availability of vaccinations or treatments for COVID‐19 (31) and in the context of potentially more virulent earlier strains (32, 33). The overall mortality rate attributable to COVID‐19 in SLE patients who received anifrolumab treatment was comparable to that reported for patients with SLE treated with biologics at the time of the COVID‐19 diagnosis (26). Of interest, 2 of the 3 patients with COVID‐related deaths were also receiving concomitant medication at the time of their AE (combined anifrolumab 300 mg group [n = 1], placebo to anifrolumab 300 mg group [n = 1]), which may have contributed to worse COVID‐19 outcomes. Importantly, there were no COVID‐related AEs in patients after they were fully vaccinated against COVID‐19, although postvaccine exposure was limited. Further data from ongoing studies are needed before conclusions can be made. These data underscore the importance of vaccination against COVID‐19 and other viral infections such as influenza and HZ in patients with SLE, especially given evidence showing vaccines to be safe and well tolerated (34, 35).
Even though this study was primarily designed to assess long‐term safety of anifrolumab in SLE, disease activity was assessed with the SLEDAI‐2K, and greater mean improvement was observed in the combined anifrolumab 300 mg group than in the combined placebo group. Improvement was sustained over time, and mean disease activity levels remained low with anifrolumab throughout the study. Treatment with anifrolumab 300 mg enabled glucocorticoid dose reduction and a lower cumulative glucocorticoid dose compared with placebo, even though there was no glucocorticoid taper required by LTE protocol. At year 4, 36.4% of anifrolumab‐treated patients were free of glucocorticoid use (0 mg/day), and 74.4% of patients were receiving a glucocorticoid dose of 0 mg/day or 0 to ≤5 mg/day, which may have contributed to overall lower prevalence of SAEs with anifrolumab. The importance of glucocorticoid reduction in SLE has been highlighted by studies showing a clear association of glucocorticoid exposure with damage accrual, osteonecrosis, cardiovascular events, and osteoporosis with fractures (36, 37). These LTE results were consistent with findings from post hoc analysis of TULIP trials that showed that anifrolumab facilitates sustained glucocorticoid tapering in patients with SLE and is associated with fewer SAEs (38).
This study is unique in that it is the first phase III, double‐blind, placebo‐controlled long‐term safety study in SLE, and it was conducted both before and during the COVID‐19 pandemic. This not only enabled initial observations of COVID‐19 viral infections in patients with SLE in both pre‐ and postvaccination periods, but also led to study disruption, with interruptions to treatment in the early part of the pandemic as well as dropouts. The current extension study also substantiates findings described in the open‐label extension study of the phase IIb MUSE trial, which previously showed safety and sustained improvement in disease activity following treatment with anifrolumab for up to 3 years in patients with moderate‐to‐severe SLE (13).
Limitations of this study include that it was not powered for statistical comparison of safety between groups, and the duration may not capture rare events with a longer latency period. Moreover, though the population in this study was typical of an SLE population, data on elderly patients are limited and pregnant patients were excluded. Post‐marketing long‐term safety assessments, including studies in pregnant and lactating women, are planned, in addition to a separate study in patients <18 years of age. Other limitations of this study include potential selection bias, as only patients completing a TULIP study, and thus potentially doing well, were eligible. Additionally, survival bias may also be a factor during the course of the LTE as no imputation was performed for patients who discontinued and/or received additional immunosuppressants, especially given the discontinuation rate observed across treatment groups, although that would favor the placebo group more than the anifrolumab group. Similarly, efficacy data could be biased based on patient withdrawals related to SLE flares. However, as DAE and flare rates were low in the LTE study, we believe that improvements in safety and efficacy observed over time cannot be explained solely by selection bias. Inclusion of placebo control in this LTE study was not considered an ethical concern, as all patients continued receiving standard care.
In conclusion, given the chronic nature of SLE and need for treatment over a prolonged time period, determining the long‐term safety and efficacy of treatments is important. The results of this 3‐year long‐term extension build upon those seen in the preceding 52‐week treatment periods of the phase III TULIP‐1 and TULIP‐2 trials and the 3‐year open‐label extension of the phase II MUSE study. These data show an acceptable long‐term safety profile of anifrolumab in SLE in addition to sustained improvements in disease activity and reduction in glucocorticoid use. Taken together, the findings support the favorable benefit–risk profile of long‐term anifrolumab as a treatment option for patients with moderate‐to‐severe SLE.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Al‐Mossawi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Kalunian, Furie, Morand, Bruce, Abreu, Hultquist, Tummala.
Acquisition of data
Hupka, Zhang, Werther, Abreu, Tummala, Lindholm.
Analysis and interpretation of the data
Kalunian, Bruce, Winthrop, Abreu, Hultquist, Tummala, Lindholm, Al‐Mossawi.
ROLE OF THE STUDY SPONSOR
AstraZeneca provided funding and medical writing assistance for the study. AstraZeneca employees were involved in the design and conduct of the study; contributed to the collection, analysis, and interpretation of the data; and supported the authors in the development of the manuscript. All authors approved the content of the submitted manuscript and were involved in the decision to submit the manuscript for publication. Publication of this article was contingent upon approval by AstraZeneca.
Supporting information
Disclosure Form
Appendix S1 Supplementary Information
ACKNOWLEDGMENTS
The authors would like to thank the investigators, research staff, health care providers, and especially the patients who participated in this study. The authors would also like to thank the clinical team, including Dr. Sule Yavuz and Will Gunther in particular for their contributions to this manuscript. Medical writing support was provided by Sheila Longo, PhD, of JK Associates Inc., part of Fishawack Health, and funded by AstraZeneca. INB is a National Institute for Health Research (NIHR) Senior Investigator Emeritus and is funded by the NIHR Manchester Biomedical Research Centre.
Listen to the podcast Here
A graphical abstract can be found in the online article at http://onlinelibrary.wiley.com/doi/10.1002/art.42392/abs.
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Supported by AstraZeneca.
A graphical abstract and author disclosures are available online at http://onlinelibrary.wiley.com/doi/10.1002/art.42392.
Footnotes
[Correction added on 9 December 2022, after first online publication: The original text stating that “all flares were mild to moderate in severity” has been changed to “the majority of flares were mild to moderate in severity.”]
Data availability
Data underlying the findings described herein may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Reuse is permitted only with permission from AstraZeneca.
REFERENCES
- 1. Justiz Vaillant AA, Goyal A, Bansal P, Varacallo M. Systemic lupus erythematosus: StatPearls Publishing, Treasure Island (FL); 2021. [PubMed] [Google Scholar]
- 2. Tanaka Y, Bae SC, Bass D, et al. Long‐term open‐label continuation study of the safety and efficacy of belimumab for up to 7 years in patients with systemic lupus erythematosus from Japan and South Korea. RMD Open 2021;7:e001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang F, Zheng J, Li Y, et al. Phase 3, long‐term, open‐label extension period of safety and efficacy of belimumab in patients with systemic lupus erythematosus in China, for up to 6 years. RMD Open 2022;8:e001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riggs JM, Hanna RN, Rajan B, et al. Characterisation of anifrolumab, a fully human anti‐interferon receptor antagonist antibody for the treatment of systemic lupus erythematosus. Lupus Sci Med 2018;5:e000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furie RA, Morand EF, Bruce IN, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP‐1): a randomised, controlled, phase 3 trial. Lancet Rheumatol 2019;1:e208–19. [DOI] [PubMed] [Google Scholar]
- 6. Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- 7. AstraZeneca Pharmaceuticals LP . SAPHNELO (anifrolumab‐fnia) [Prescribing Information]. 2021. URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761123s000lbl.pdf.
- 8. AstraZeneca . Saphnelo approved in Japan for systemic lupus erythematosus; 2021. URL: https://www.astrazeneca.com/media-centre/press-releases/2021/saphnelo-approved-in-japan-for-sle.html.
- 9. AstraZeneca . Saphnelo recommended for approval in the EU by CHMP for the treatment of patients with systemic lupus erythematosus; 2021. URL: https://www.astrazeneca.com/media-centre/press-releases/2021/saphnelo-recommended-for-eu-approval-for-sle.html.
- 10. AstraZeneca Pharmaceuticals LP . Australian Product Information. Saphnelo®; 2022. URL: https://www.guildlink.com.au/gc/ws/astra/pi.cfm?product=appsaphn10322. [Google Scholar]
- 11. Burki TK. FDA approval for anifrolumab in patients with lupus. Lancet Rheumatol 2021;3:e689. [Google Scholar]
- 12. Furie R, Khamashta M, Merrill JT, et al. Anifrolumab, an anti–interferon‐α receptor monoclonal antibody, in moderate‐to‐severe systemic lupus erythematosus. Arthritis Rheumatol 2017;69:376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chatham WW, Furie R, Saxena A, et al. Long‐term safety and efficacy of anifrolumab in adults with systemic lupus erythematosus: results of a phase II open‐label extension study. Arthritis Rheumatol 2021;73:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morand EF, Furie RA, Bruce IN, et al. Efficacy of anifrolumab across organ domains in patients with moderate‐to‐severe systemic lupus erythematosus: a posthoc analysis of pooled data from the TULIP‐1 and TULIP‐2 trials. Lancet Rheumatol 2022;4:e282–92. [DOI] [PubMed] [Google Scholar]
- 15. ClinicalTrials.gov . Long term safety of anifrolumab in adult subjects with active systemic lupus erythematosus (TULIP SLE LTE NCT02794285). URL: https://clinicaltrials.gov/ct2/show/NCT02794285.
- 16. Ghebreyesus TA. WHO director‐general's opening remarks at the media briefing on COVID‐19—11 March 2020. URL: https://www.who.int/director‐general/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19—11‐march‐2020.
- 17. Maharani W, Ratnaningsih DF, Utami F, et al. Activity disease in SLE patients affected IFN‐γ in the IGRA results. J Inflamm Res 2020;13:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tummala R, Abreu G, Pineda L, et al. Safety profile of anifrolumab in patients with active SLE: an integrated analysis of phase II and III trials. Lupus Sci Med 2021;8:e000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furie R, Morand EF, Askanase AD, et al. Anifrolumab reduces flare rates in patients with moderate to severe systemic lupus erythematosus. Lupus 2021;30:1254–63. [DOI] [PubMed] [Google Scholar]
- 20. Strand V, O'Quinn S, Furie RA, et al. Clinical meaningfulness of a British Isles lupus assessment group‐based composite lupus assessment response in terms of patient‐reported outcomes in moderate to severe systemic lupus erythematosus: a post‐hoc analysis of the phase 3 TULIP‐1 and TULIP‐2 trials of anifrolumab. Lancet Rheumatol 2022;4:e198–207. [DOI] [PubMed] [Google Scholar]
- 21. GSK . Shingrix approved in the US for prevention of shingles in immunocompromised adults; 2021. URL: https://www.drugs.com/newdrugs/shingrix‐approved‐us‐prevention‐shingles‐immunocompromised‐adults‐5612.html.
- 22. van Vollenhoven RF, Navarra SV, Levy RA, et al. Long‐term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a Phase III study extension. Rheumatology (Oxford) 2020;59:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casey KA, Guo X, Smith MA, et al. Type I interferon receptor blockade with anifrolumab corrects innate and adaptive immune perturbations of SLE. Lupus Sci Med 2018;5:e000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aringer M, Alarcon‐Riquelme ME, Clowse M, et al. A glimpse into the future of systemic lupus erythematosus. Ther Adv Musculoskelet Dis 2022;14:1759720X221086719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Notz Q, Meybohm P, Kranke P, et al. Antirheumatic drugs, B cell depletion and critical COVID‐19: correspondence on ‘Clinical course of coronavirus disease 2019 (COVID‐19) in a series of 17 patients with systemic lupus erythematosus under long‐term treatment with hydroxychloroquine' by Mathian et al. Ann Rheum Dis 2020;81:e216. [DOI] [PubMed] [Google Scholar]
- 26. Ugarte‐Gil MF, Alarcón GS, Izadi Z, et al. Characteristics associated with poor COVID‐19 outcomes in individuals with systemic lupus erythematosus: data from the COVID‐19 Global Rheumatology Alliance. Ann Rheum Dis 2022;81:970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fernandez‐Ruiz R, Paredes JL, Niewold TB. COVID‐19 in patients with systemic lupus erythematosus: lessons learned from the inflammatory disease. Transl Res 2021;232:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathian A, Mahevas M, Rohmer J, et al. Clinical course of coronavirus disease 2019 (COVID‐19) in a series of 17 patients with systemic lupus erythematosus under long‐term treatment with hydroxychloroquine. Ann Rheum Dis 2020;79:837–9. [DOI] [PubMed] [Google Scholar]
- 29. Pan H, Peto R, Henao‐Restrepo AM, et al. Repurposed antiviral drugs for Covid‐19: Interim WHO Solidarity Trial results. N Engl J Med 2021;384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life‐threatening COVID‐19. Science 2020;370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid‐19. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meng B, Abdullahi A, Ferreira I, et al. Altered TMPRSS2 usage by SARS‐CoV‐2 Omicron impacts infectivity and fusogenicity. Nature 2022;603:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki R, Yamasoba D, Kimura I, et al. Attenuated fusogenicity and pathogenicity of SARS‐CoV‐2 Omicron variant. Nature 2022;603:700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Felten R, Kawka L, Dubois M, et al. Tolerance of COVID‐19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatol 2021;3:e613–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang CC, Chang YS, Chen WS, et al. Effects of annual influenza vaccination on morbidity and mortality in patients with systemic lupus erythematosus: a nationwide cohort study. Sci Rep 2016;6:37817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ugarte‐Gil MF, Mak A, Leong J, et al. Impact of glucocorticoids on the incidence of lupus‐related major organ damage: a systematic literature review and meta‐regression analysis of longitudinal observational studies. Lupus Sci Med 2021;8:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruce IN, O'Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruce IN, van Vollenhoven RF, Morand EF, et al. Sustained glucocorticoid tapering in the phase 3 trials of anifrolumab: a post‐hoc analysis of the TULIP‐1 and TULIP‐2 trials. Rheumatology (Oxford) 2022:keac491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1 Supplementary Information
Data Availability Statement
Data underlying the findings described herein may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Reuse is permitted only with permission from AstraZeneca.


